 The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
 The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
 The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
 The Manufacturing Process For Biological Drugs Download Scientific Diagram
The Manufacturing Process For Biological Drugs Download Scientific Diagram
Http Www Cee Org Tep Lab Bench Pdf 2017 Ml Talk Pdf
 Biologics Production Impact Of Bioburden Contaminations Of Non Sterile Process Intermediates On Patient Safety And Product Quality American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Biologics Production Impact Of Bioburden Contaminations Of Non Sterile Process Intermediates On Patient Safety And Product Quality American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 The Manufacturing Process For Biological Drugs Download Scientific Diagram
The Manufacturing Process For Biological Drugs Download Scientific Diagram
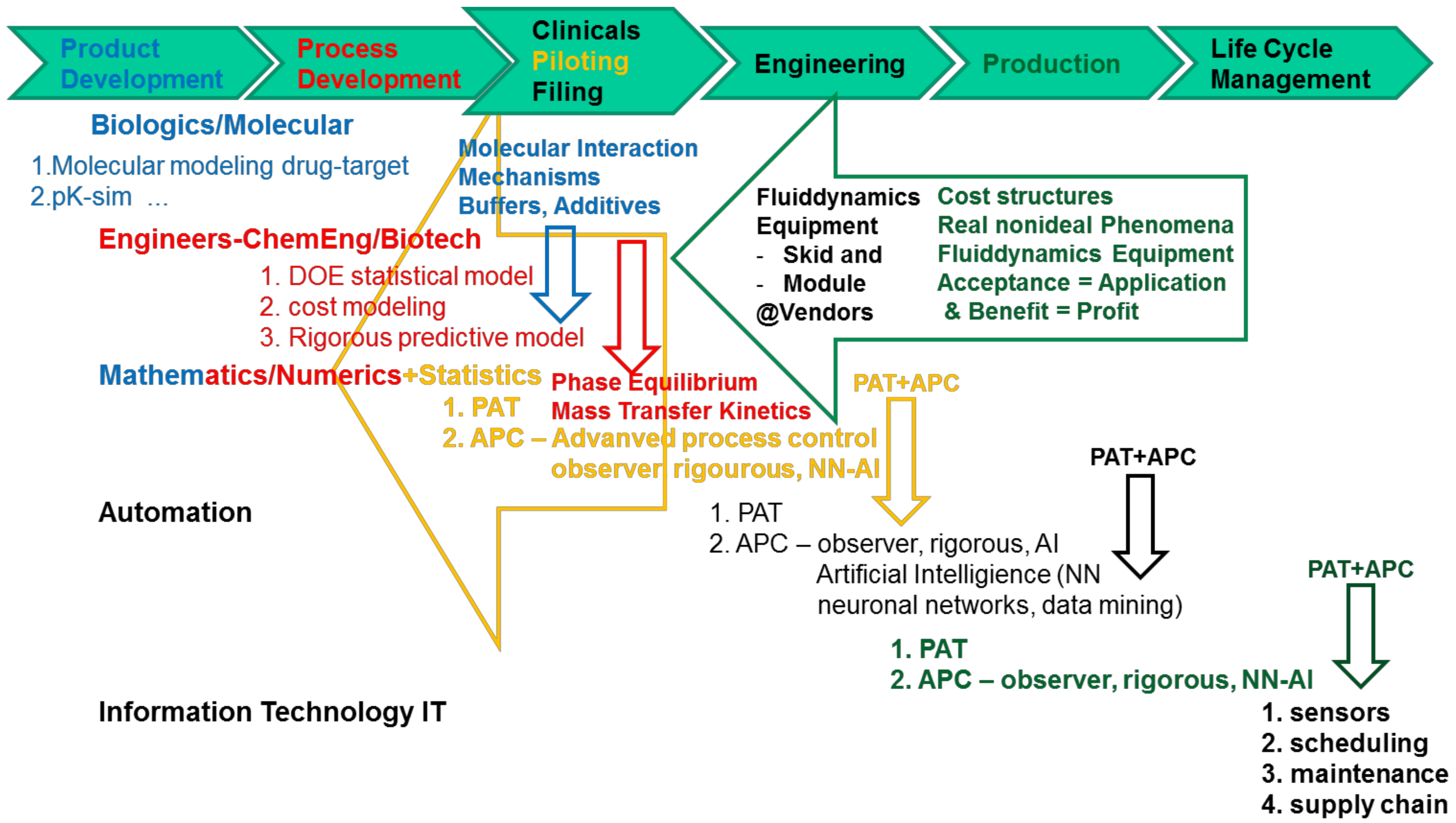 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 The Use Of Thorough Microbial Impact Assessment To Control Bioburden On A Particular Process Step During Manufacturing Of Biologic Products American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
The Use Of Thorough Microbial Impact Assessment To Control Bioburden On A Particular Process Step During Manufacturing Of Biologic Products American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Figure 26 3 From The Biomanufacturing Of Biotechnology Products Semantic Scholar
Figure 26 3 From The Biomanufacturing Of Biotechnology Products Semantic Scholar
 A Single Use Strategy To Enable Manufacturing Of Affordable Biologics Sciencedirect
A Single Use Strategy To Enable Manufacturing Of Affordable Biologics Sciencedirect
 The Biopharmaceutical Manufacturing Technology Flowchart Exemplifying Download Scientific Diagram
The Biopharmaceutical Manufacturing Technology Flowchart Exemplifying Download Scientific Diagram

 Practical Considerations For Doe Implementation In Quality By Design Bioprocess Internationalbioprocess International
Practical Considerations For Doe Implementation In Quality By Design Bioprocess Internationalbioprocess International
 Figure 26 3 From The Biomanufacturing Of Biotechnology Products Semantic Scholar
Figure 26 3 From The Biomanufacturing Of Biotechnology Products Semantic Scholar
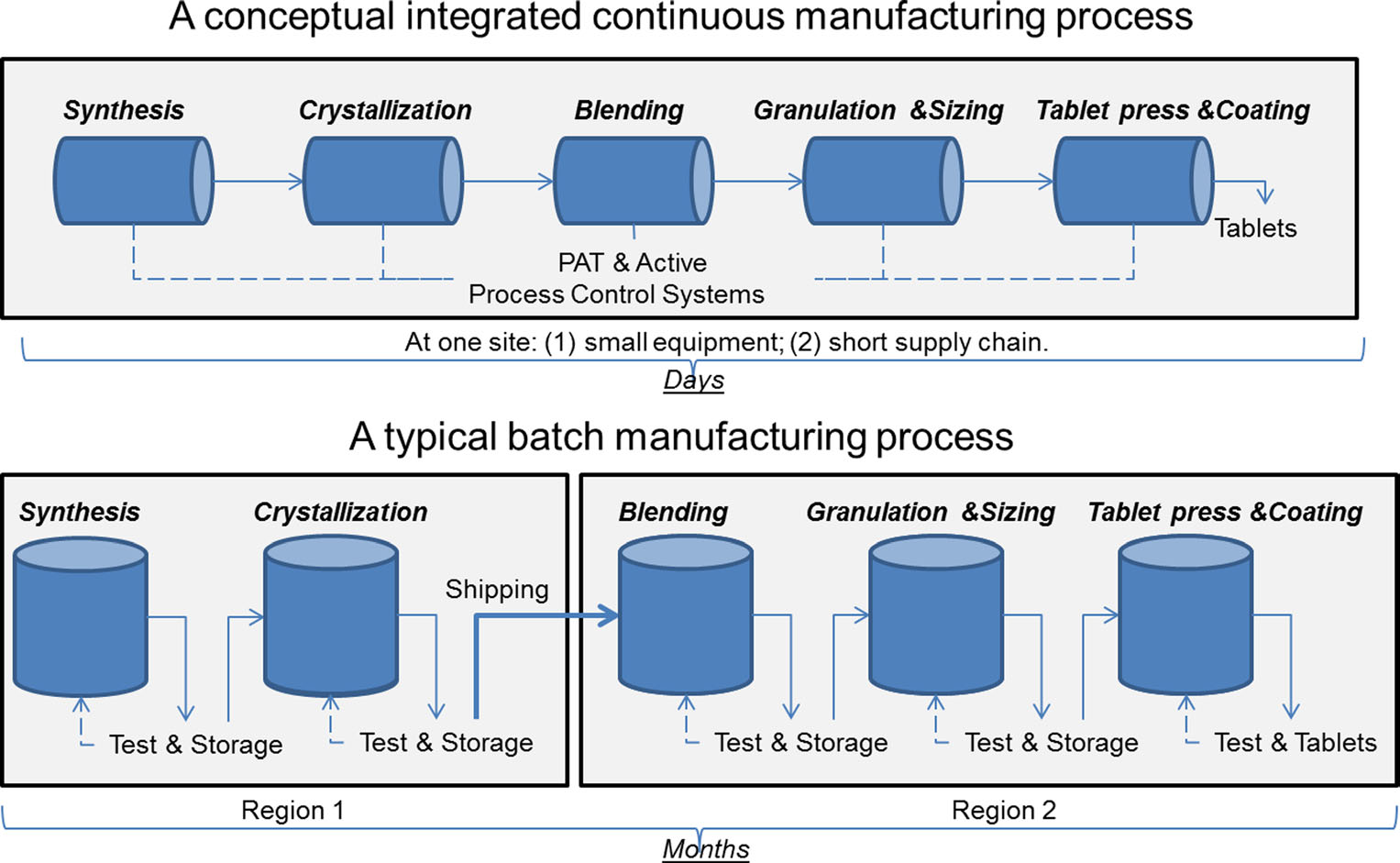
 Biomanufacturing How Biologics Are Made Biotech Primer Weekly
Biomanufacturing How Biologics Are Made Biotech Primer Weekly
 Biologics Production Impact Of Bioburden Contaminations Of Non Sterile Process Intermediates On Patient Safety And Product Quality American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Biologics Production Impact Of Bioburden Contaminations Of Non Sterile Process Intermediates On Patient Safety And Product Quality American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Chapter 79 Pharmaceutical Industry
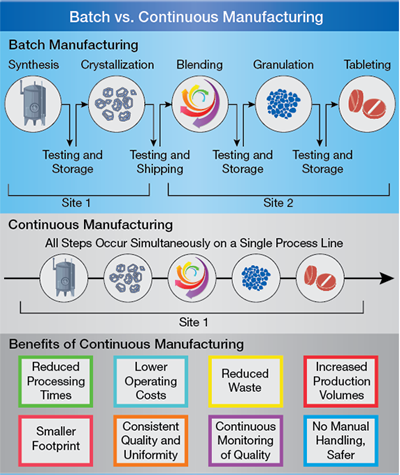 Pharmaceutical Manufacturing Current Trends And What S Next Aiche
Pharmaceutical Manufacturing Current Trends And What S Next Aiche
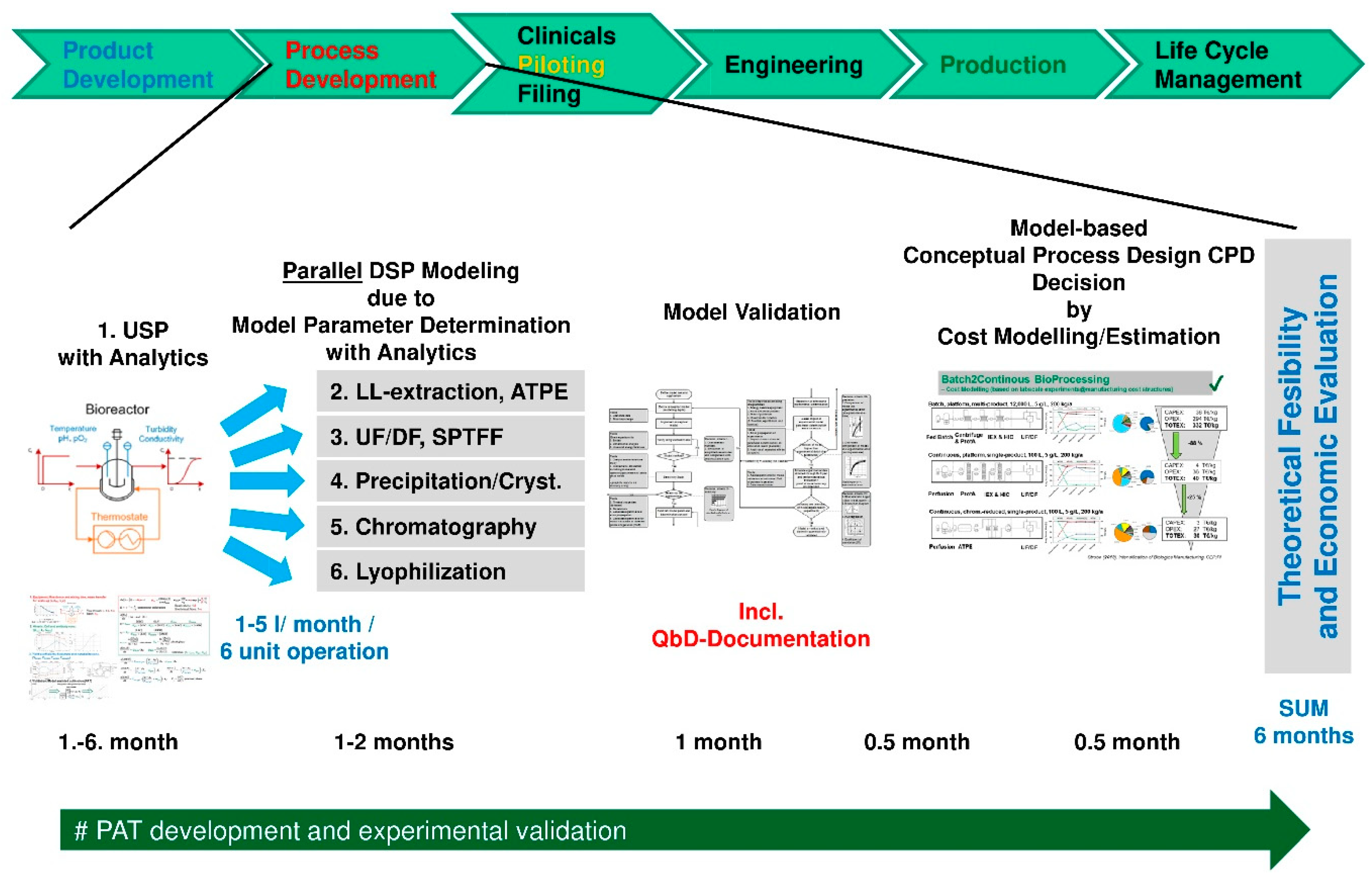 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 Implementation Of Qbd For Manufacturing Of Biologics Has It Met The Expectations Sciencedirect
Implementation Of Qbd For Manufacturing Of Biologics Has It Met The Expectations Sciencedirect
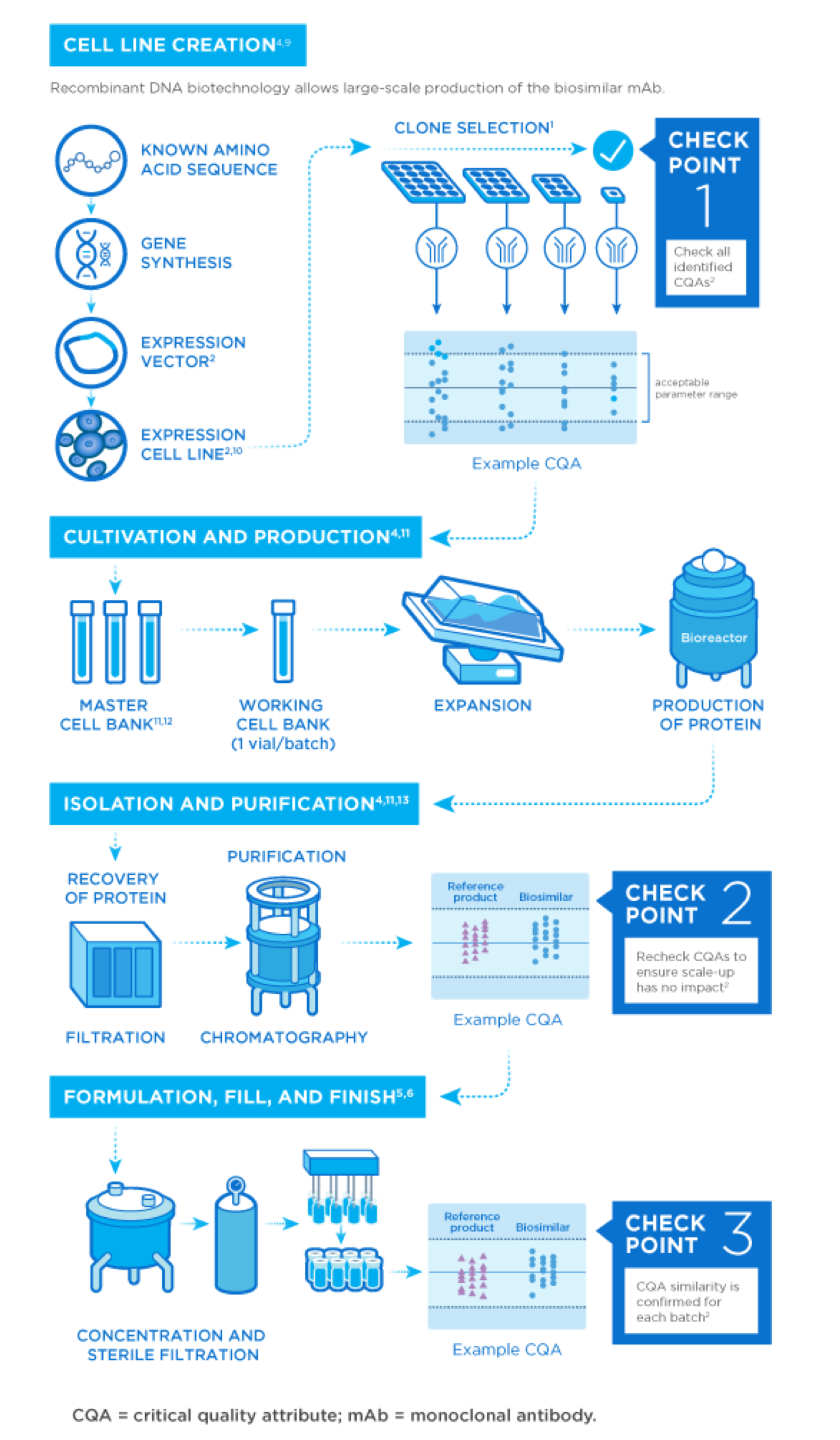 Next Generation Biologics Manufacturing Amgen Biosimilars
Next Generation Biologics Manufacturing Amgen Biosimilars
1st Asean Overview Workshop On Gmp For Biologicals Biosimilars 2018 Report Gabi Journal
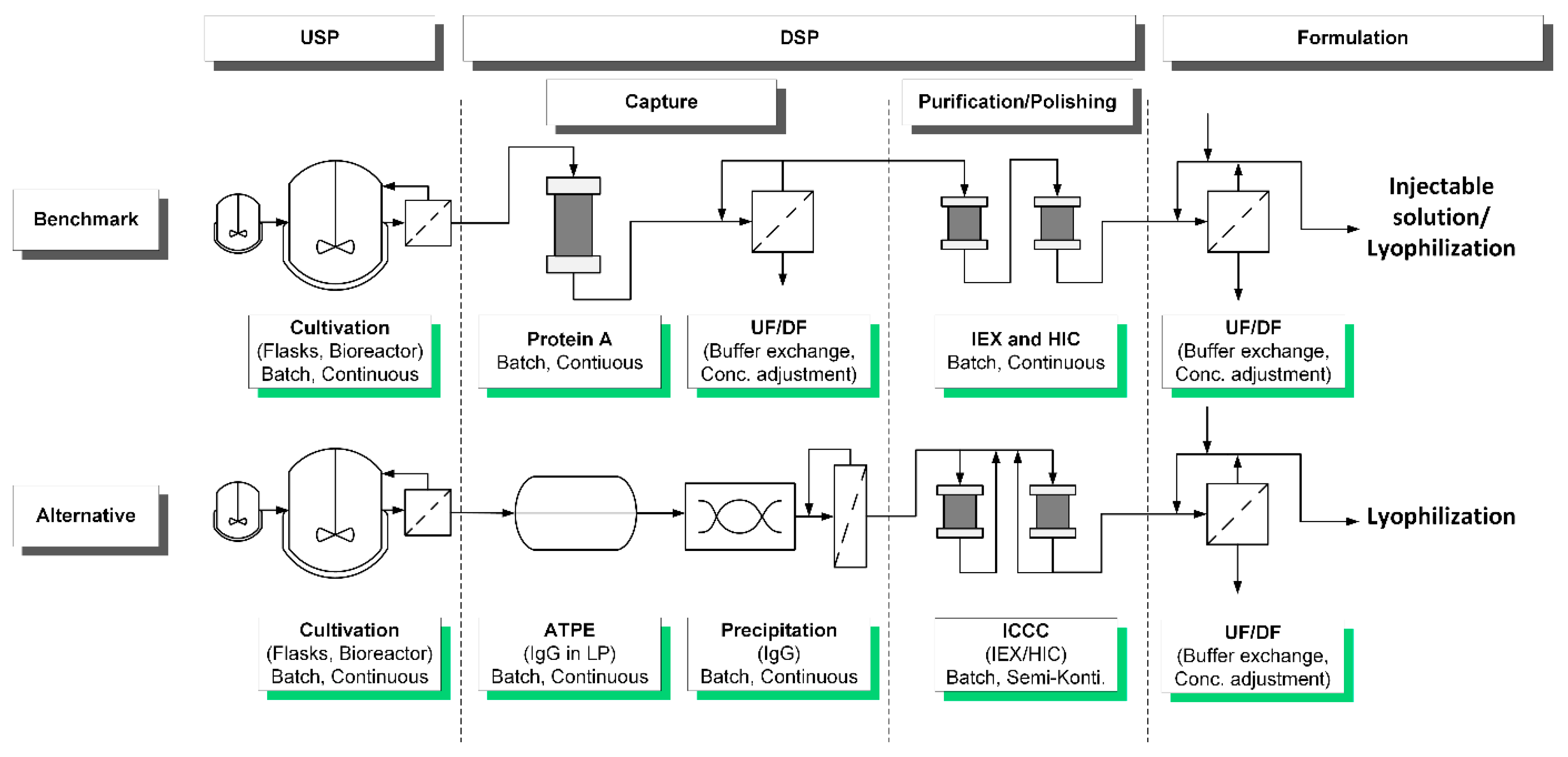 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Process Integration Of Precipitation In Mab Downstream Processing
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Process Integration Of Precipitation In Mab Downstream Processing
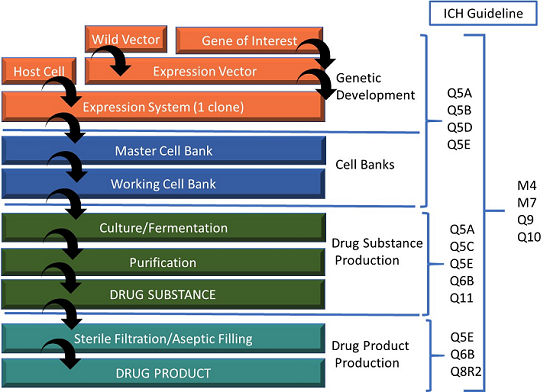 Considerations For Biologic Drug Substance And Drug Product Testing
Considerations For Biologic Drug Substance And Drug Product Testing
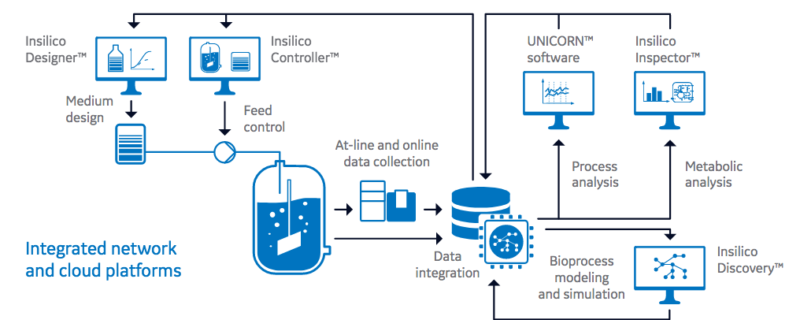 Implementing Digital Biomanufacturing In Process Development
Implementing Digital Biomanufacturing In Process Development
Http Cdn Intechopen Com Pdfs 37168 Intech Biological Products Manufacturing Handling Packaging And Storage Pdf
 Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Critical Factors For Fill Finish Manufacturing Of Biologics Bioprocess Internationalbioprocess International
Critical Factors For Fill Finish Manufacturing Of Biologics Bioprocess Internationalbioprocess International
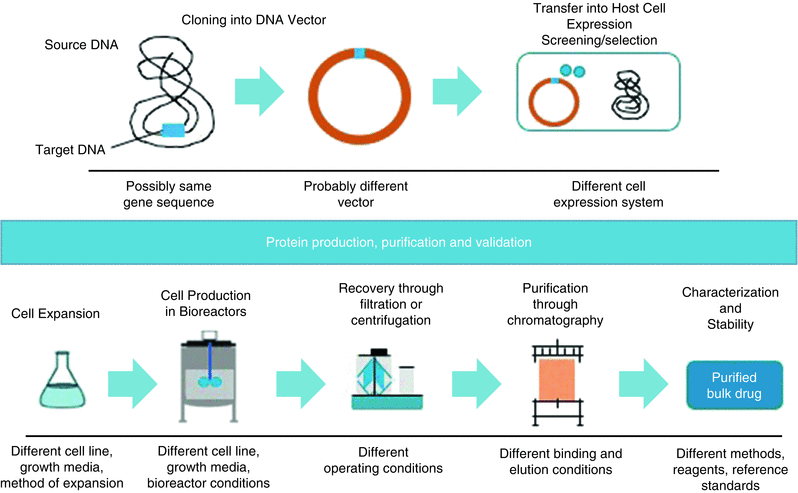 Manufacturing Of Biologics Springerlink
Manufacturing Of Biologics Springerlink
 Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
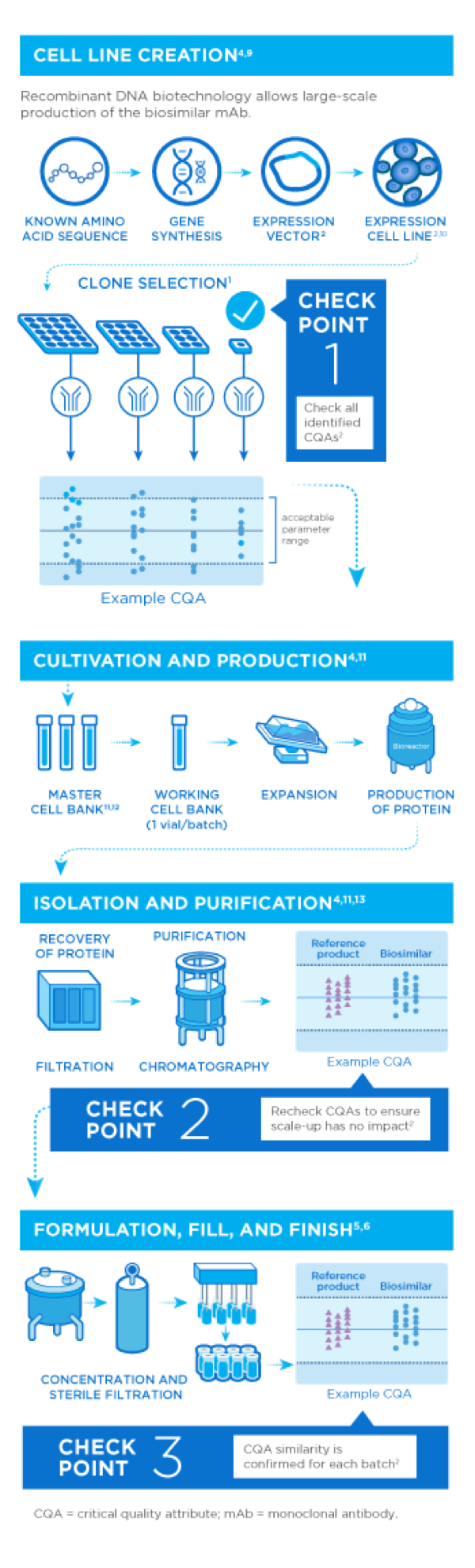 Next Generation Biologics Manufacturing Amgen Biosimilars
Next Generation Biologics Manufacturing Amgen Biosimilars
 The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
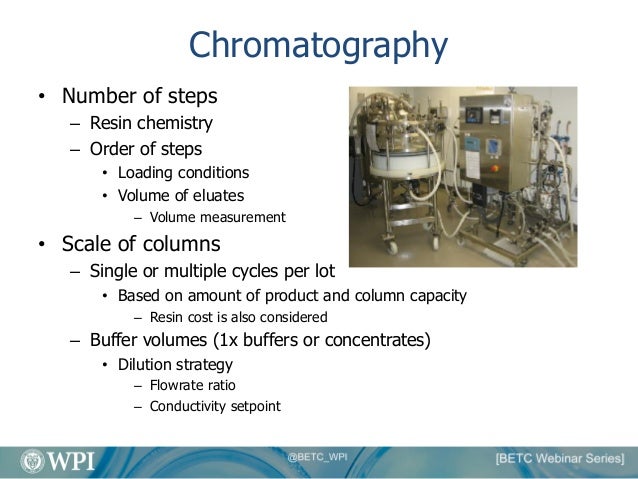 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
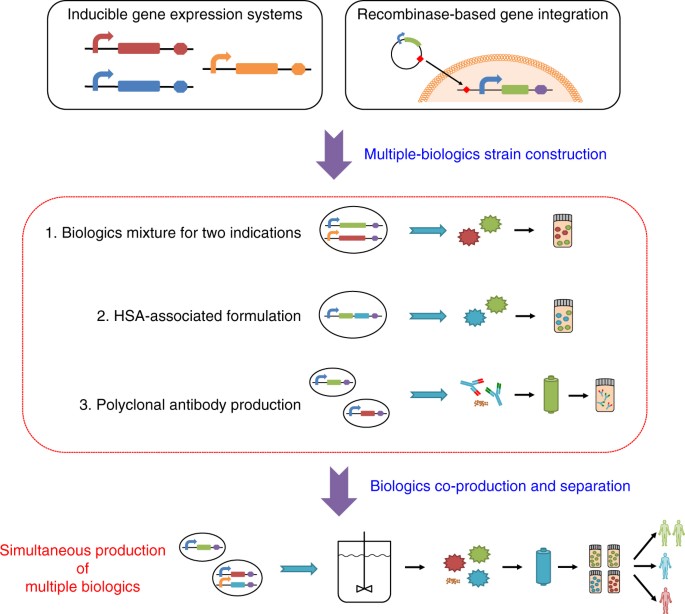
 Versatile And On Demand Biologics Co Production In Yeast Nature Communications
Versatile And On Demand Biologics Co Production In Yeast Nature Communications
 Automation Of A Single Use Final Bulk Filtration Step Enhancing Operational Flexibility And Facilitating Compliant Right First Time Manufacturingbioprocess International
Automation Of A Single Use Final Bulk Filtration Step Enhancing Operational Flexibility And Facilitating Compliant Right First Time Manufacturingbioprocess International
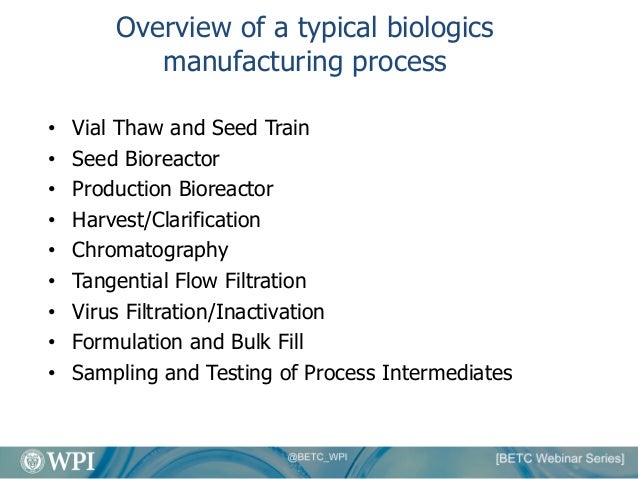 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
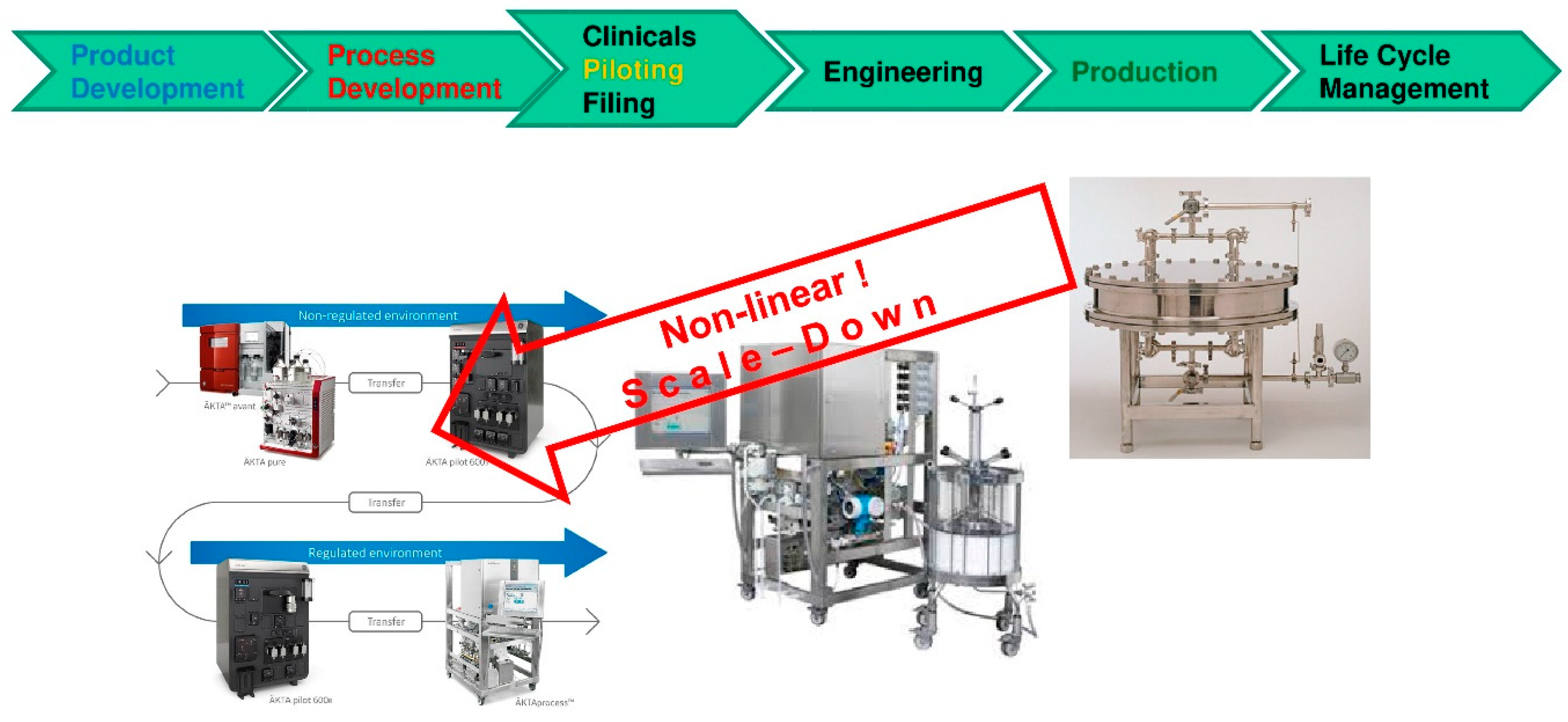 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 The Biomanufacturing Of Biotechnology Products Sciencedirect
The Biomanufacturing Of Biotechnology Products Sciencedirect
 Manufacturing Cell Therapies The Paradigm Shift In Health Care Of This Century National Academy Of Medicine
Manufacturing Cell Therapies The Paradigm Shift In Health Care Of This Century National Academy Of Medicine
 Liposomal Drug Product Manufacturing Process Flow Diagrams A Batch Download Scientific Diagram
Liposomal Drug Product Manufacturing Process Flow Diagrams A Batch Download Scientific Diagram
 Process Analytical Technologies And Data Analytics For The Manufacture Of Monoclonal Antibodies Trends In Biotechnology
Process Analytical Technologies And Data Analytics For The Manufacture Of Monoclonal Antibodies Trends In Biotechnology
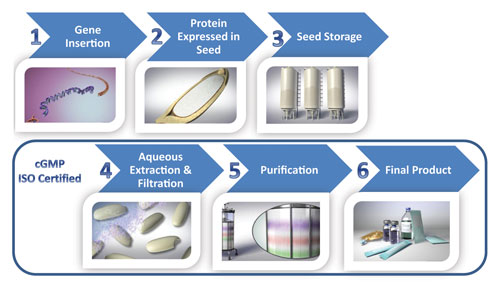 Plant Based Protein Biomanufacturing
Plant Based Protein Biomanufacturing
Biologics Businesses Annual Report 2018
 Scheduling And Optimization Of Biomanufacturing Activities In Multi Product Facilities With Disposable Technologies Biopharma Asia
Scheduling And Optimization Of Biomanufacturing Activities In Multi Product Facilities With Disposable Technologies Biopharma Asia
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrydhsxmbz2r0wvra Cahgwjzwqnlg Sv4q9hgqccvcegcyyli Usqp Cau
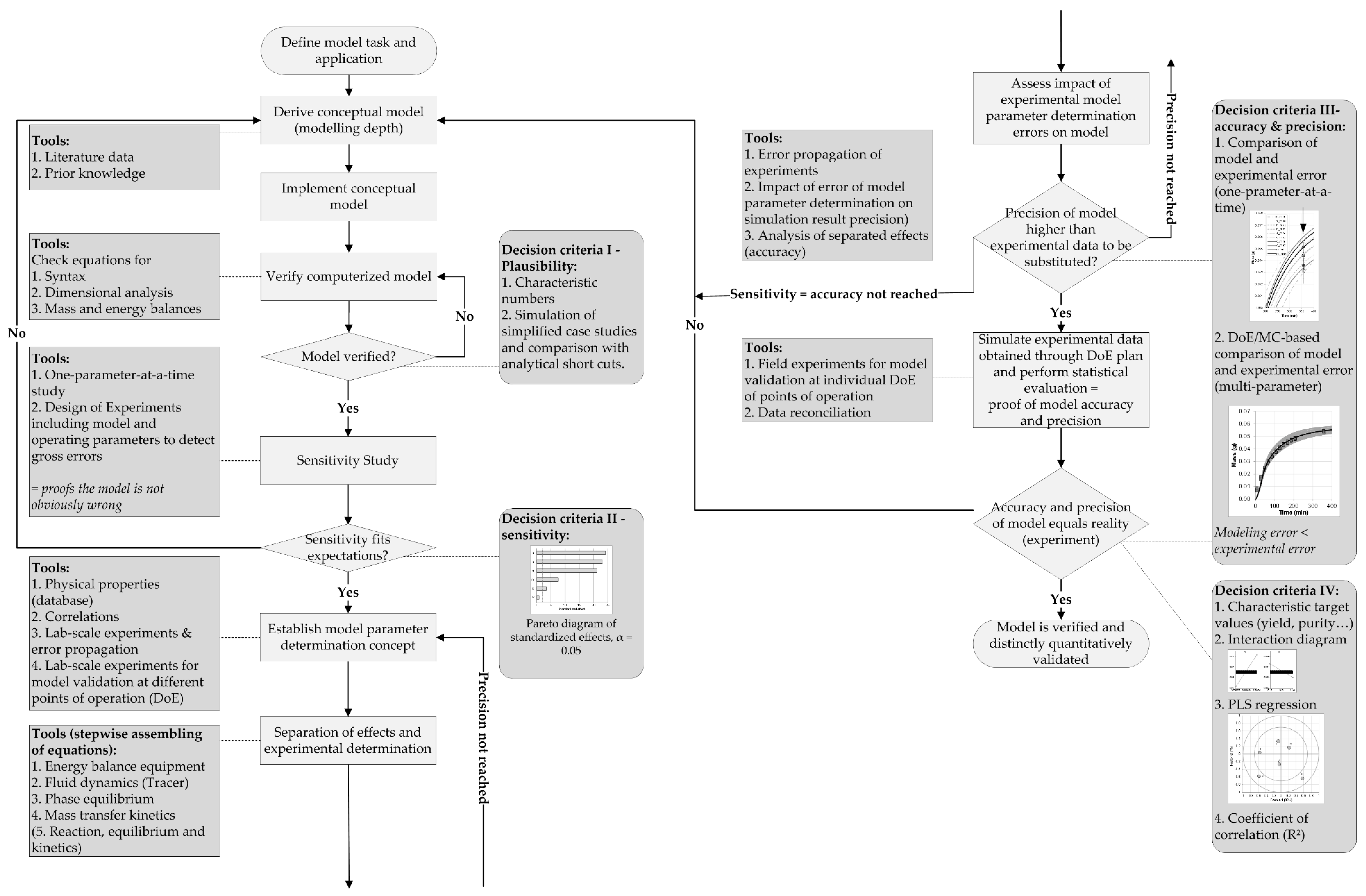 Processes Free Full Text Accelerating Biologics Manufacturing By Upstream Process Modelling Html
Processes Free Full Text Accelerating Biologics Manufacturing By Upstream Process Modelling Html
 Improving Mab Manufacturing Productivity By Optimizing Buffer And Media Prep Process Flow Avantor
Improving Mab Manufacturing Productivity By Optimizing Buffer And Media Prep Process Flow Avantor
 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
 A Single Use Strategy To Enable Manufacturing Of Affordable Biologics Sciencedirect
A Single Use Strategy To Enable Manufacturing Of Affordable Biologics Sciencedirect

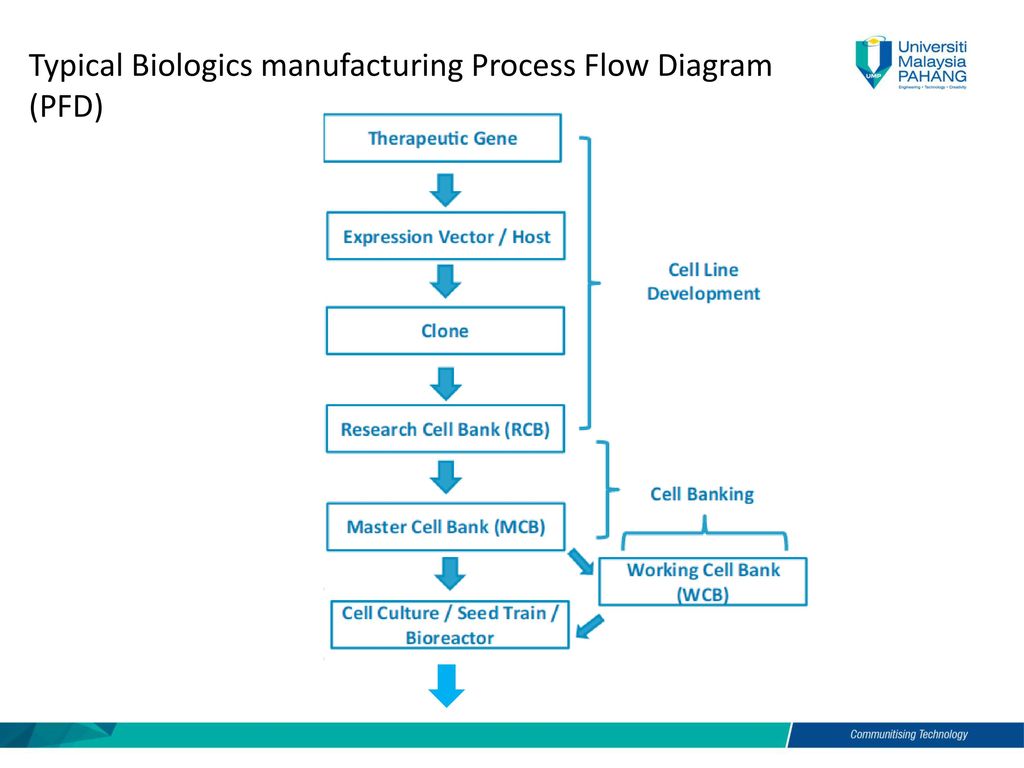 Bsb Biomanufacturing Chapter 3 Biomanufacturing Processes Ppt Video Online Download
Bsb Biomanufacturing Chapter 3 Biomanufacturing Processes Ppt Video Online Download
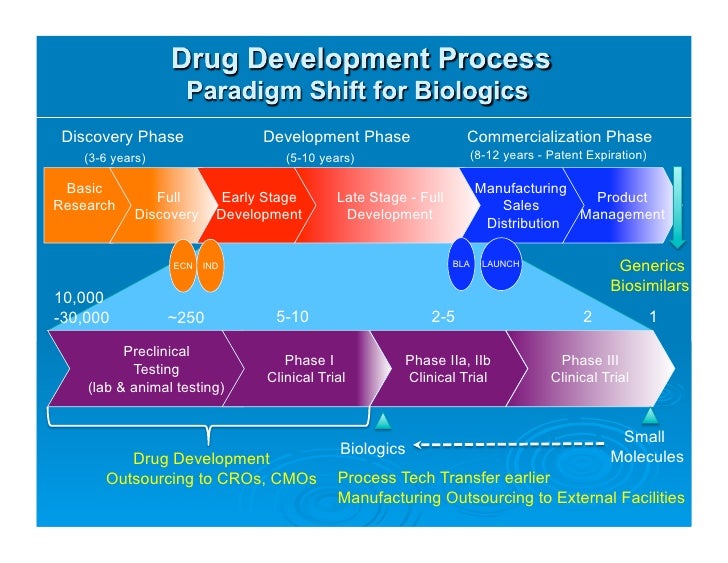 As Process Development And Manufacturing Cmc For Biologics Developm
As Process Development And Manufacturing Cmc For Biologics Developm
 Implementation Of A Platform Approach For Early Biologics Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Implementation Of A Platform Approach For Early Biologics Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Chapter 79 Pharmaceutical Industry
 Optimizing Media Flow In Biologics Manufacturing
Optimizing Media Flow In Biologics Manufacturing
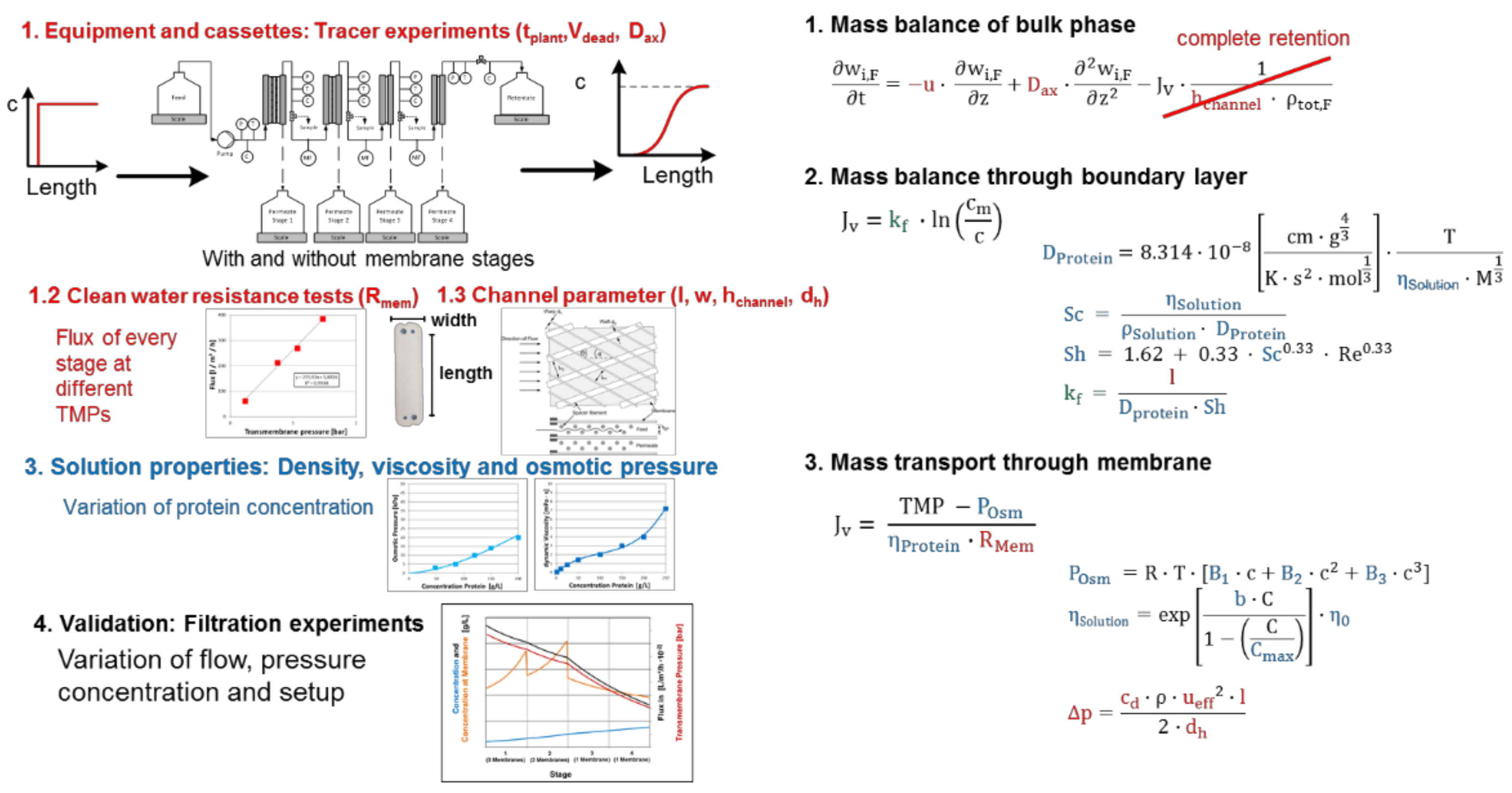 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
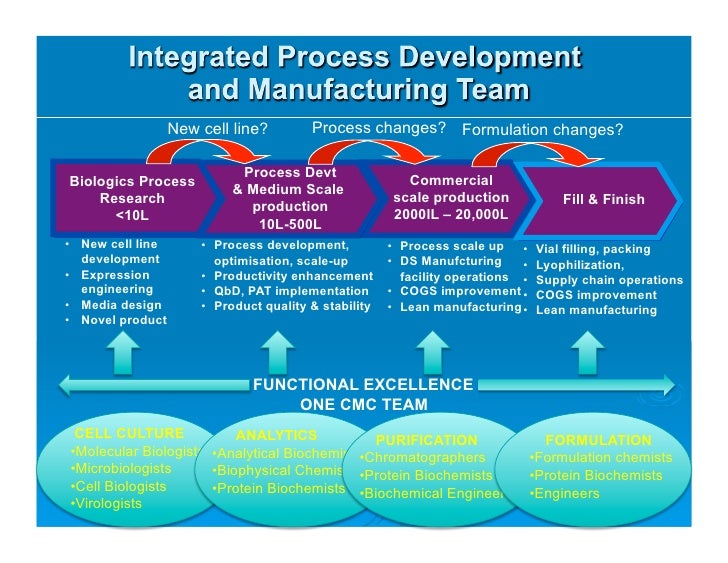 As Process Development And Manufacturing Cmc For Biologics Developm
As Process Development And Manufacturing Cmc For Biologics Developm
 The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
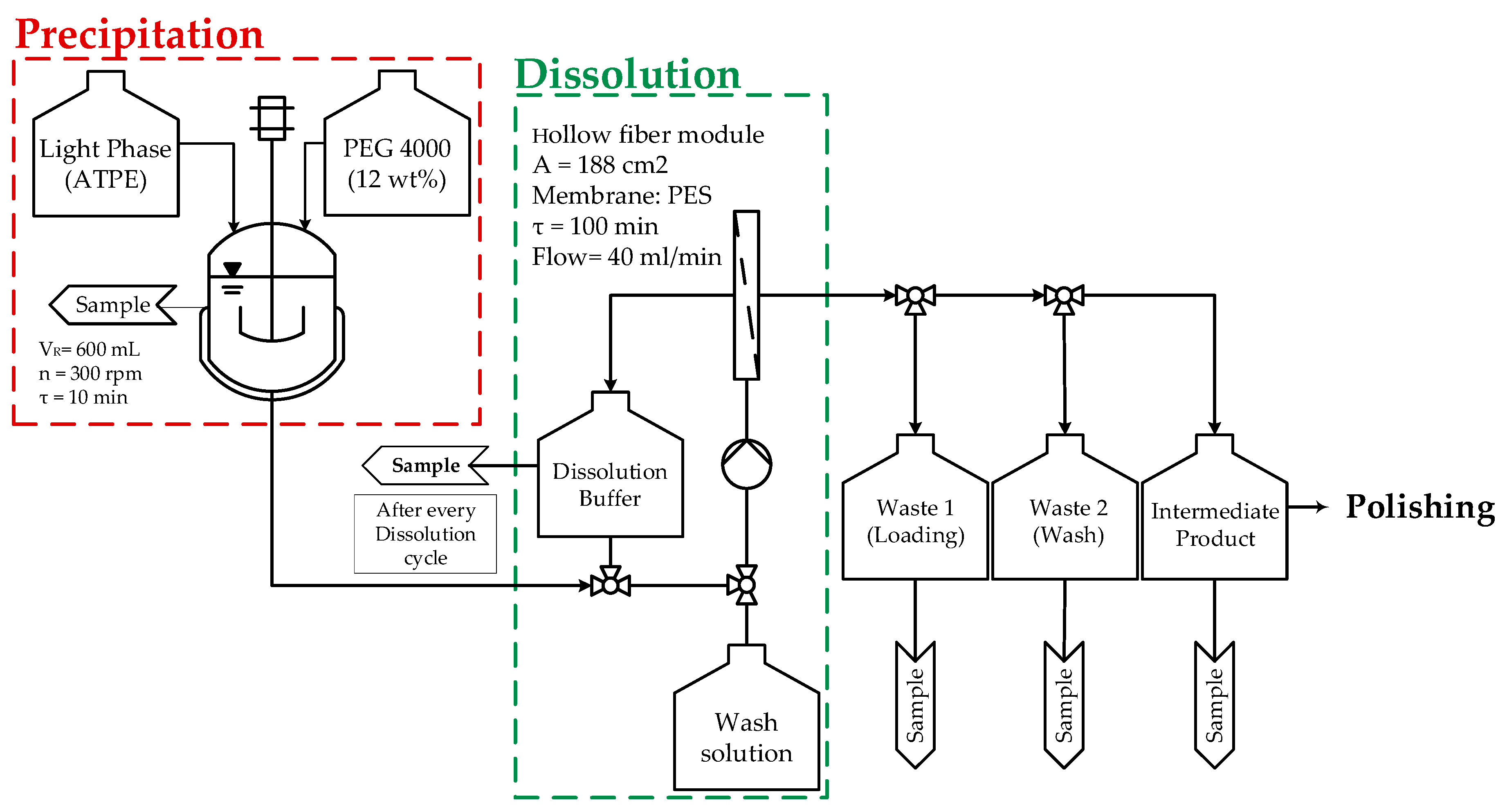 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Process Integration Of Precipitation In Mab Downstream Processing
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Process Integration Of Precipitation In Mab Downstream Processing
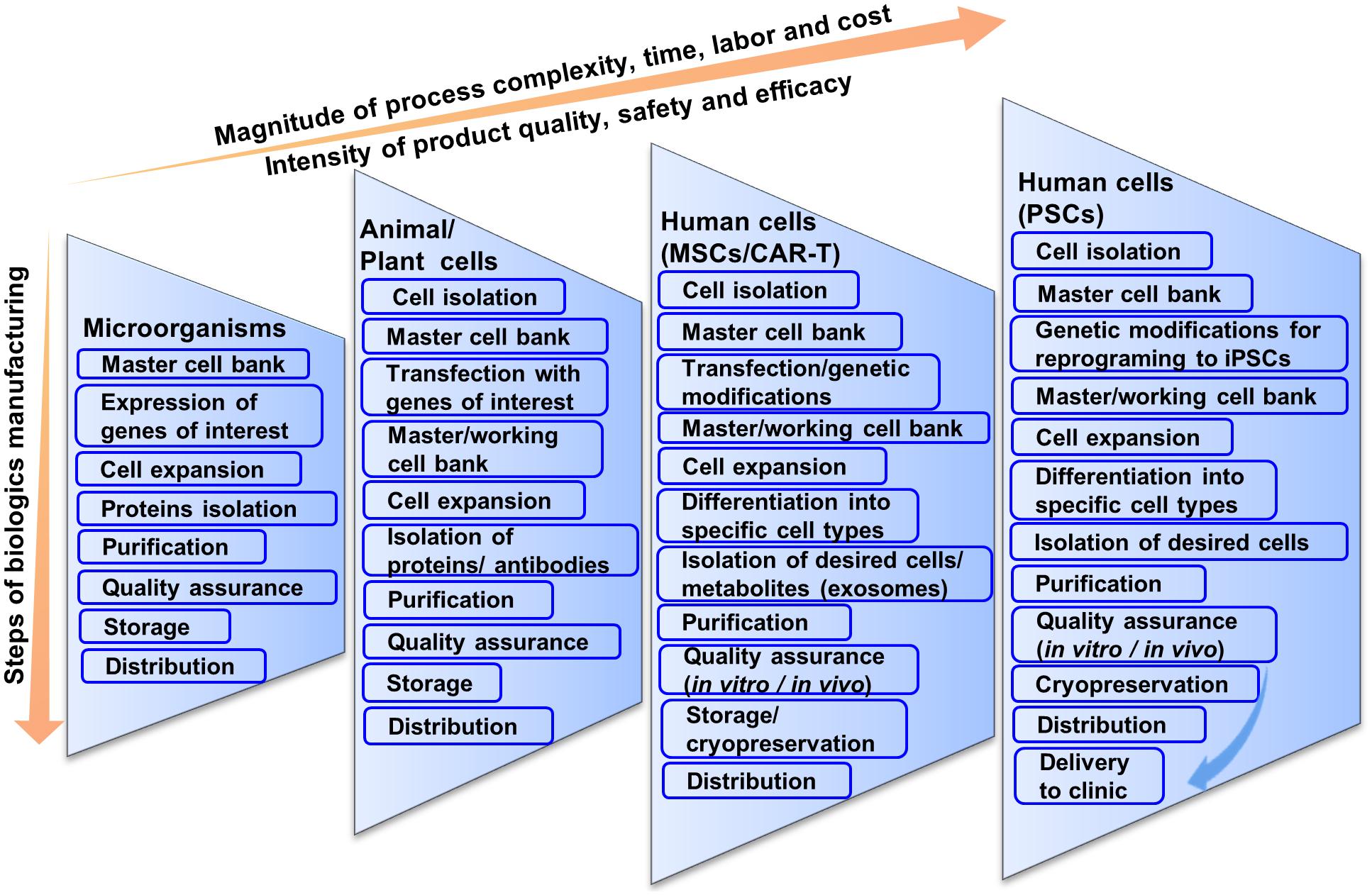 Frontiers Cell Based Therapy Manufacturing In Stirred Suspension Bioreactor Thoughts For Cgmp Compliance Bioengineering And Biotechnology
Frontiers Cell Based Therapy Manufacturing In Stirred Suspension Bioreactor Thoughts For Cgmp Compliance Bioengineering And Biotechnology
 Biologics Production Impact Of Bioburden Contaminations Of Non Sterile Process Intermediates On Patient Safety And Product Quality American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Biologics Production Impact Of Bioburden Contaminations Of Non Sterile Process Intermediates On Patient Safety And Product Quality American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Biosimilar Development Biosimilar Biological Products Development Applications
Biosimilar Development Biosimilar Biological Products Development Applications
Http Nvi Ddc Moph Go Th Download Ectd Module 201 9 20mar 1 Manufacturing 20process 20of 20biological 20products 2006032016 Pdf
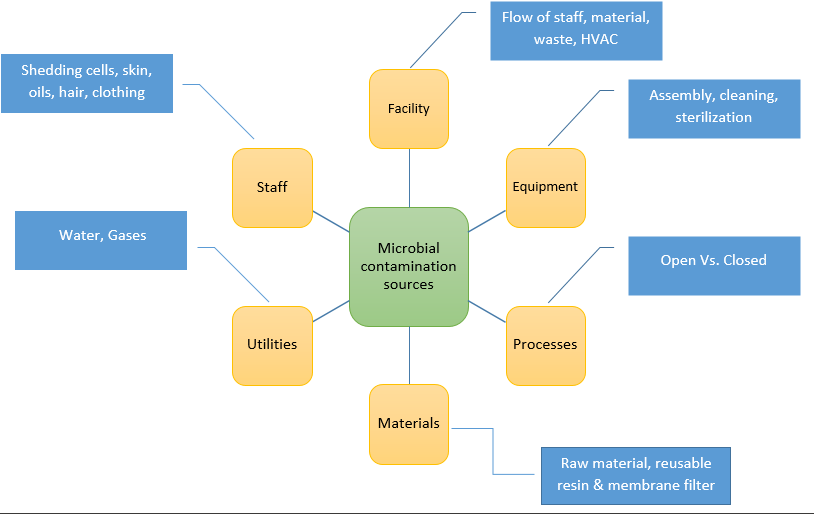
 Biosimilar Development Biosimilar Biological Products Development Applications
Biosimilar Development Biosimilar Biological Products Development Applications
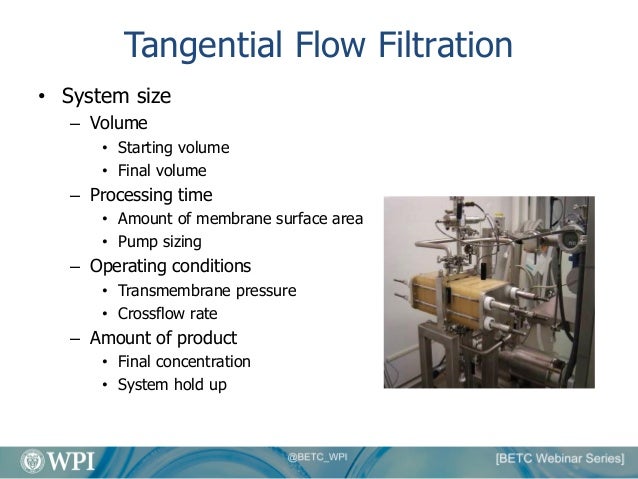 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
Https Www Ema Europa Eu En Documents Presentation Presentation Manufacturing Process Biologics Kowid Ho Afssaps En Pdf
 Process Flow Diagram Showing The Major Unit Operations And Most Of The Download Scientific Diagram
Process Flow Diagram Showing The Major Unit Operations And Most Of The Download Scientific Diagram
 Process Intensification In Biologics Manufacturing Sciencedirect
Process Intensification In Biologics Manufacturing Sciencedirect
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrpdxjcdervch0upv04yqighiztko9nbqkldio9g0pki0hj6vo7 Usqp Cau
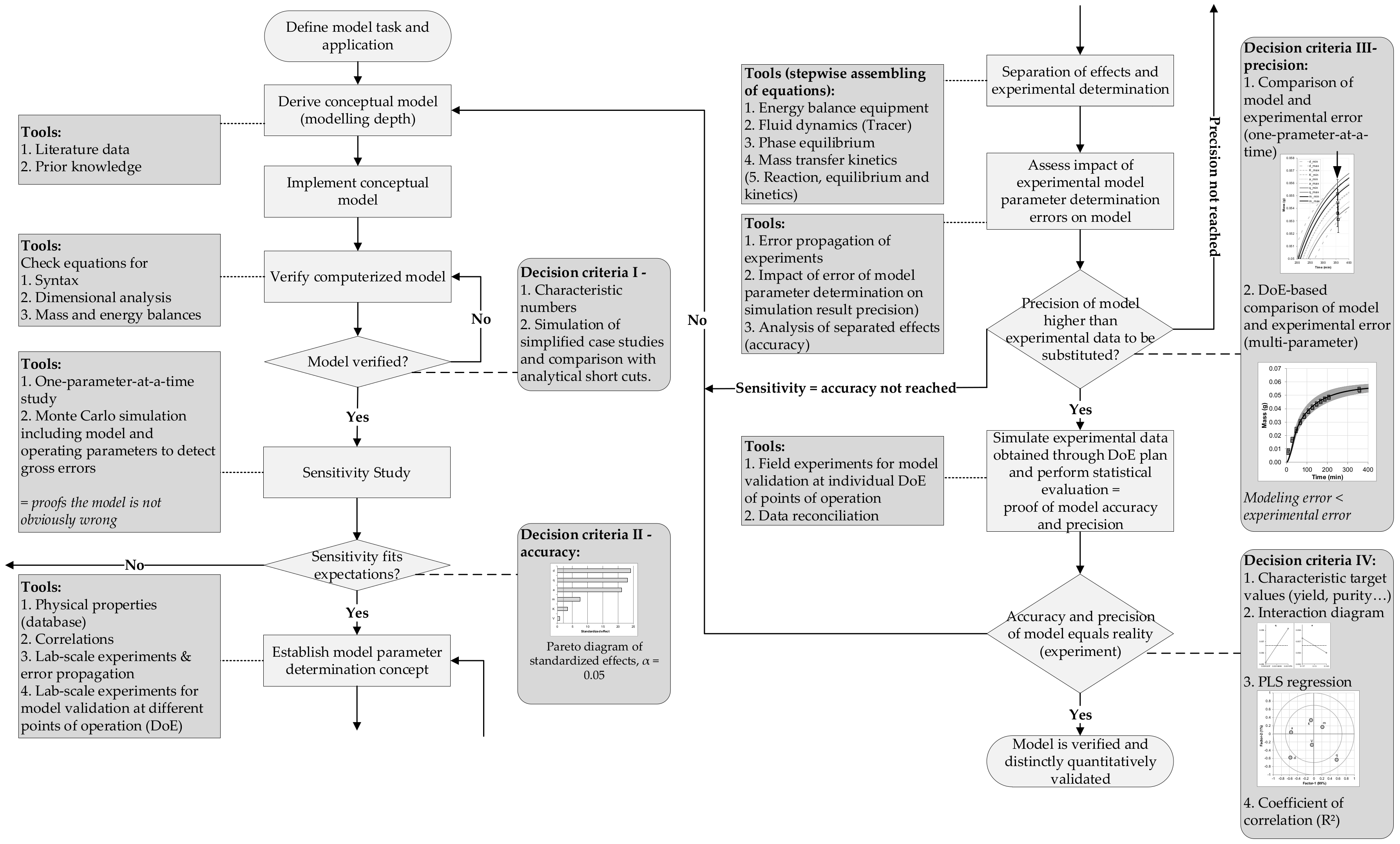 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 Analysis Of Cost Of Cell Therapy Manufacturing Autologous Cell Therapies Part 1bioprocess International
Analysis Of Cost Of Cell Therapy Manufacturing Autologous Cell Therapies Part 1bioprocess International
 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
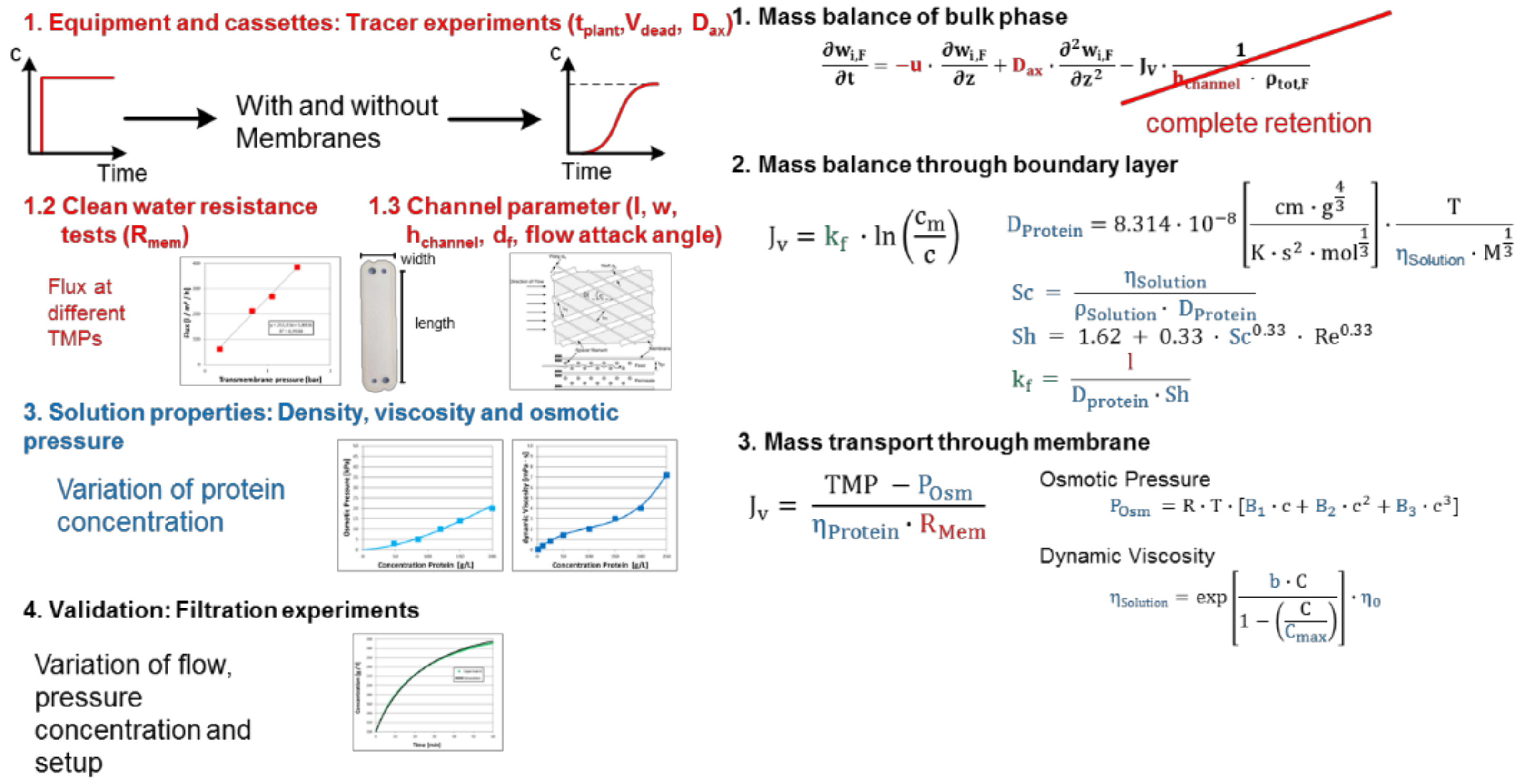 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 How Are Biosimilars Developed Fresenius Kabi Biosimilars
How Are Biosimilars Developed Fresenius Kabi Biosimilars
 Standardized Economic Cost Modeling For Next Generation Mab Production Bioprocess Internationalbioprocess International
Standardized Economic Cost Modeling For Next Generation Mab Production Bioprocess Internationalbioprocess International
Antibody Purification Process Development And Manufacturing Biopharm International
 Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Steps To Ensure Adequate Supply Of Biological Medicines Considerations For The Healthcare Provider Gabi Journal
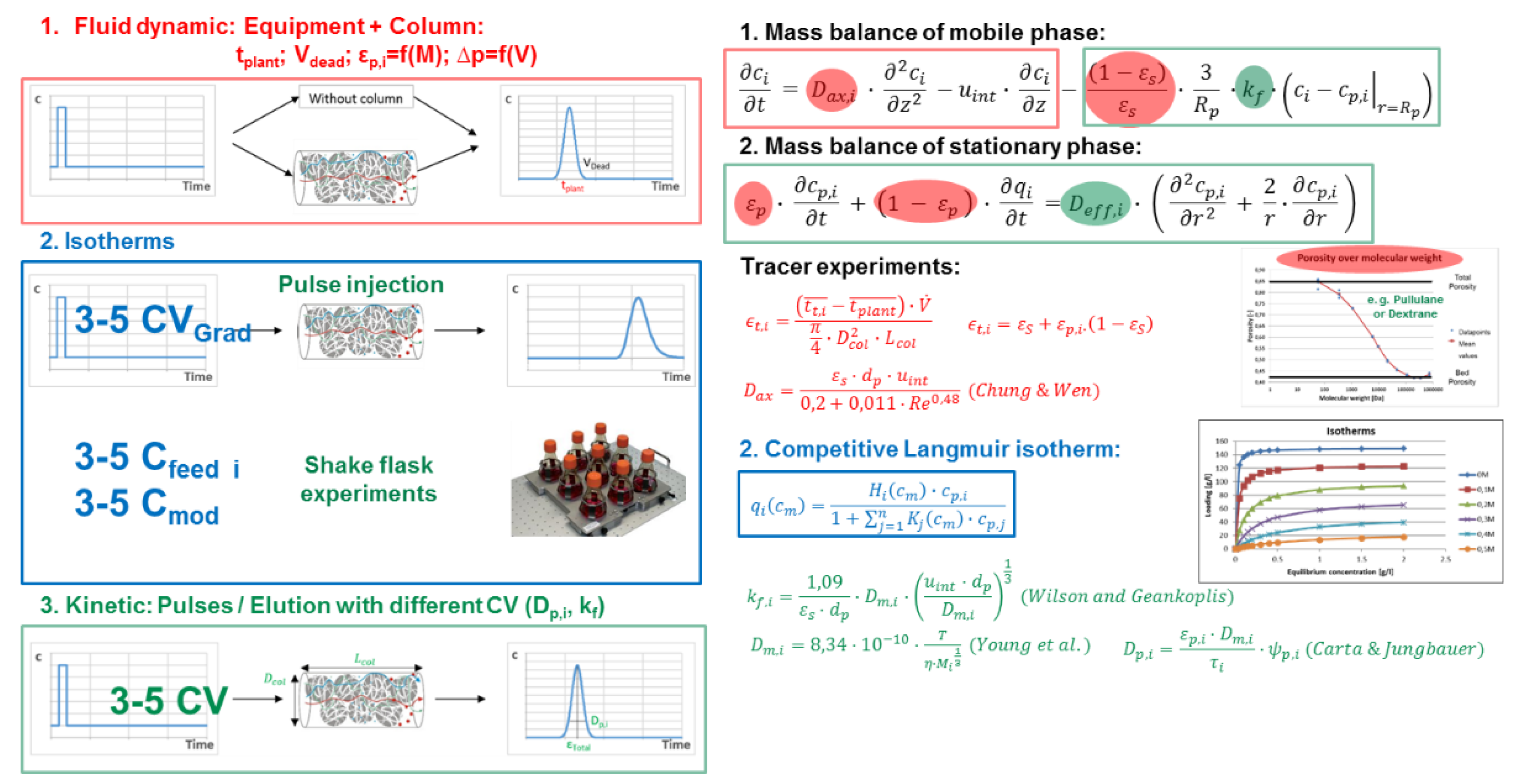 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Chapter 79 Pharmaceutical Industry
 Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Downstream Process Dsp Engineering Flow Diagram Of The Integrated Download Scientific Diagram
Downstream Process Dsp Engineering Flow Diagram Of The Integrated Download Scientific Diagram
 Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Continuous Manufacturing Progress And The Bio Pharmaceutical Industry Reality Or Fad American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 The Biomanufacturing Of Biotechnology Products Sciencedirect
The Biomanufacturing Of Biotechnology Products Sciencedirect
 Drivers Opportunities And Limits Of Continuous Processing Bioprocess Internationalbioprocess International
Drivers Opportunities And Limits Of Continuous Processing Bioprocess Internationalbioprocess International
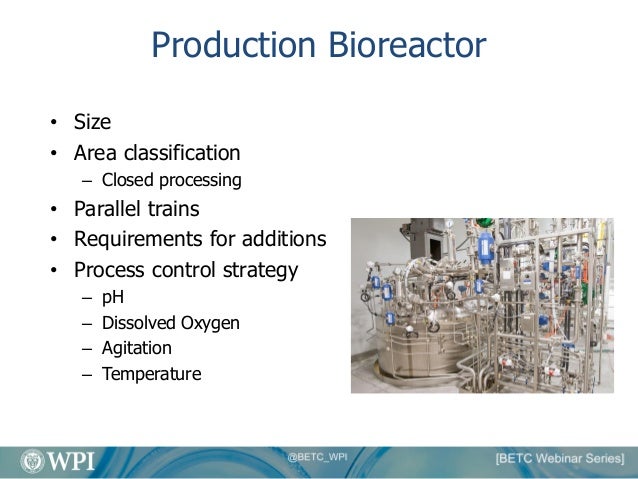 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
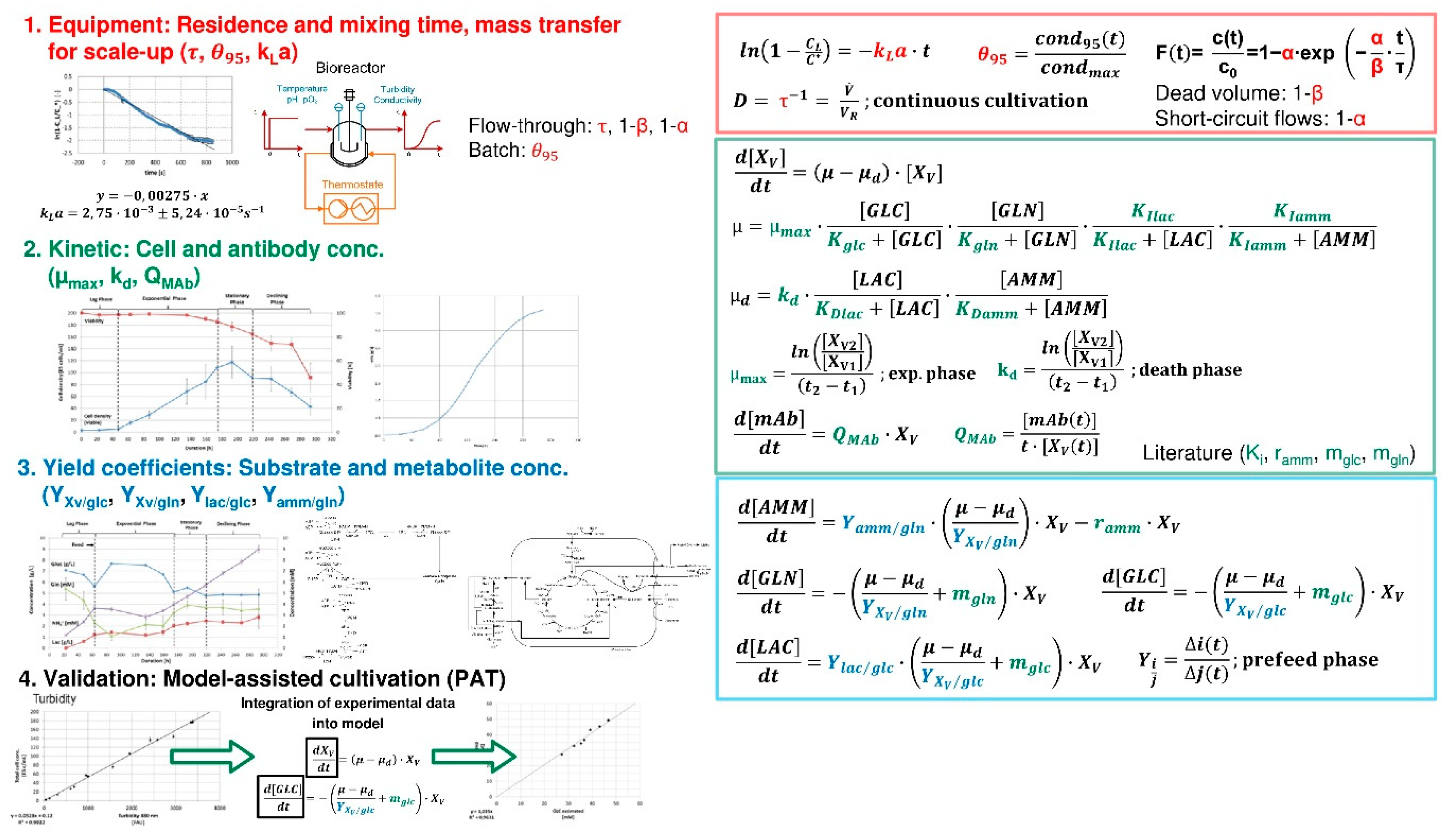 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
 Pdf Development Of Biosimilars
Pdf Development Of Biosimilars
Http Biomanufacturing Org Uploads Files 212662612262892472 Chapter 2 Pdf
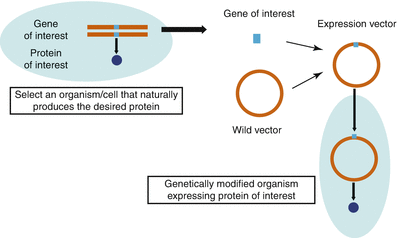 Manufacturing Of Biologics Springerlink
Manufacturing Of Biologics Springerlink
 Production Of Fos From Enzymatic Transformation Of Sucrose Industrial Processes For Functional Ingredients Novasep
Production Of Fos From Enzymatic Transformation Of Sucrose Industrial Processes For Functional Ingredients Novasep
Https Cytovance Com S Chapter26 Biotechnology Entrepreneurship Pdf
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcsoxa6epba8arurit7rmrsjb69vkq Tkzasww1qhgrvagbzehsn Usqp Cau
 Aws And Novartis Re Inventing Pharma Manufacturing Aws For Industries
Aws And Novartis Re Inventing Pharma Manufacturing Aws For Industries
Proceedings Of A Workshop Continuous Manufacturing For The Modernization Of Pharmaceutical Production Proceedings Of A Workshop The National Academies Press
 Press Releases Successful Cgmp Conjugation Of A Novel Adc For Phase I Clinical Trials
Press Releases Successful Cgmp Conjugation Of A Novel Adc For Phase I Clinical Trials
 Biopharma Services Eurofins Scientific
Biopharma Services Eurofins Scientific
 Reviewed by guruku
on
February 16, 2021
Rating:
Reviewed by guruku
on
February 16, 2021
Rating:



No comments: