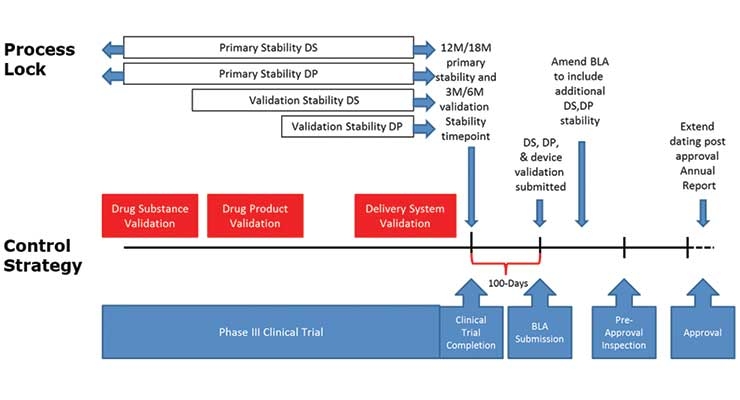
 Process Validation In Biologics Development Contract Pharma
Process Validation In Biologics Development Contract Pharma
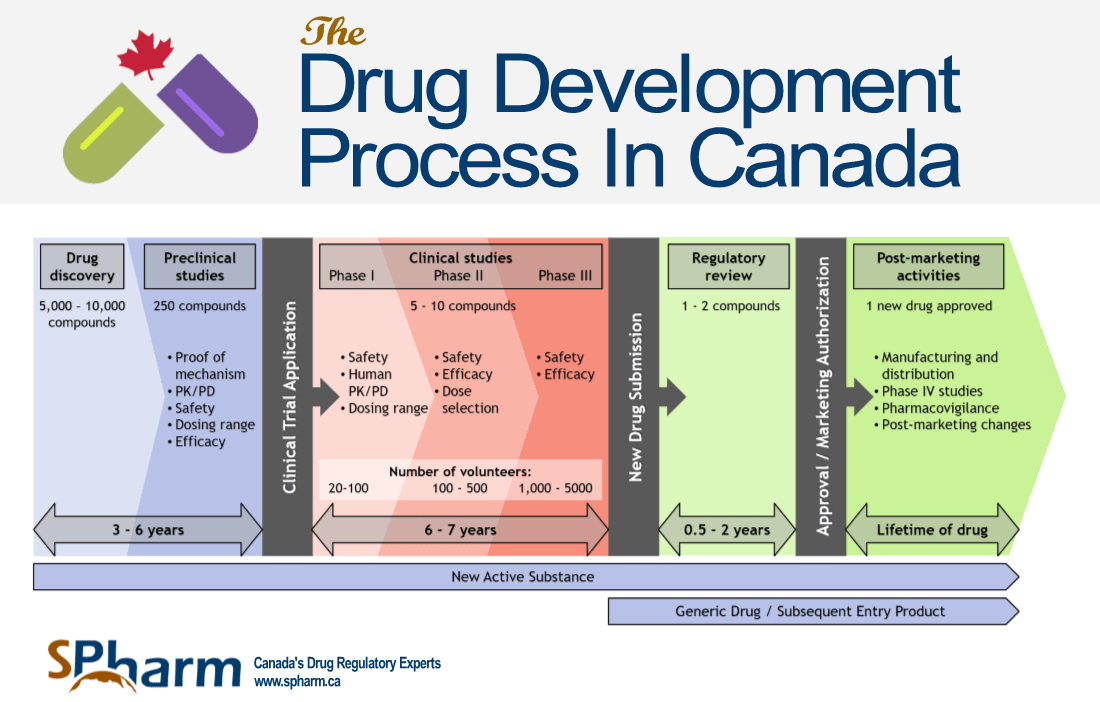
 Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
 Implementation Of A Platform Approach For Early Biologics Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Implementation Of A Platform Approach For Early Biologics Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
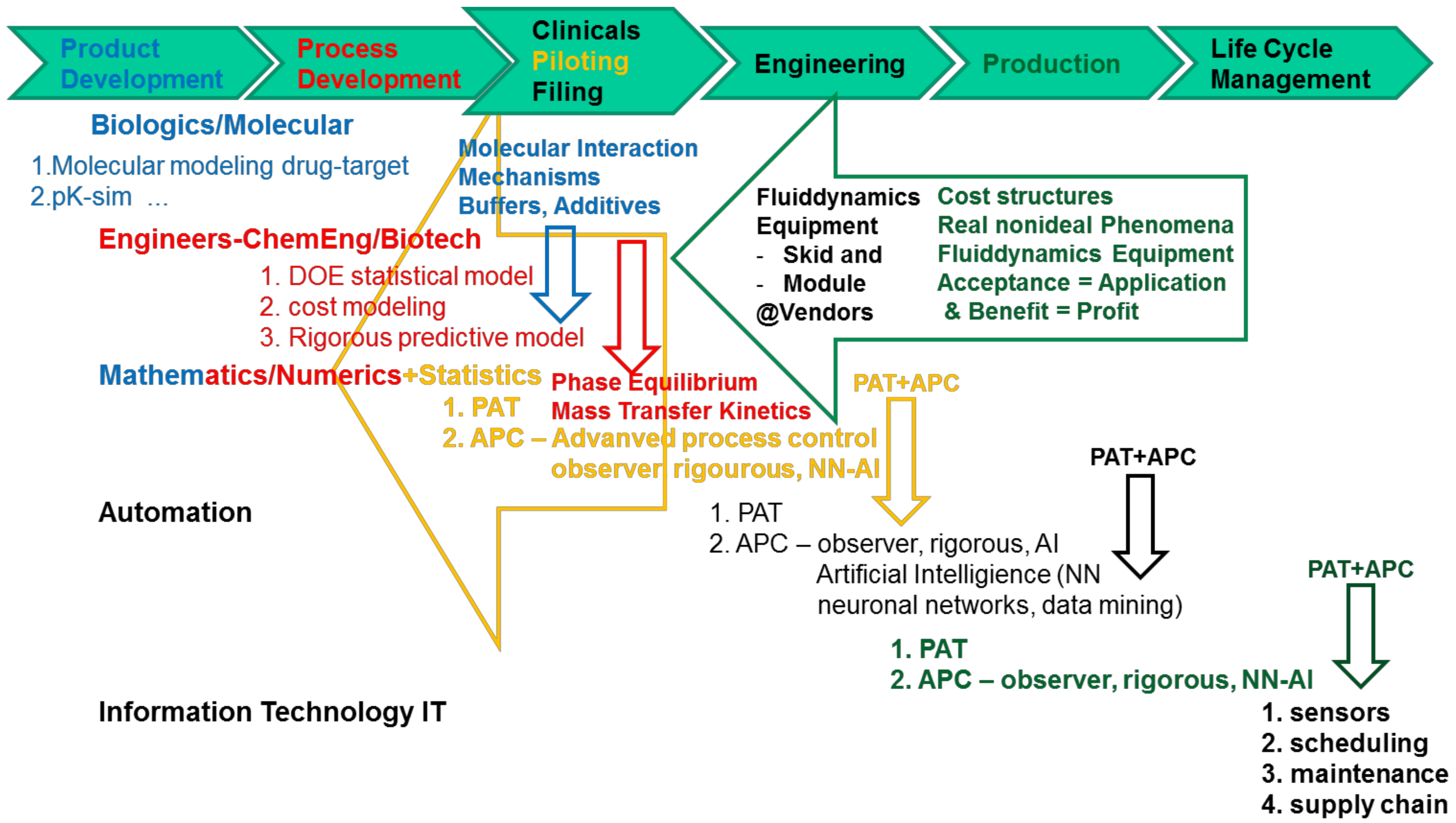 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
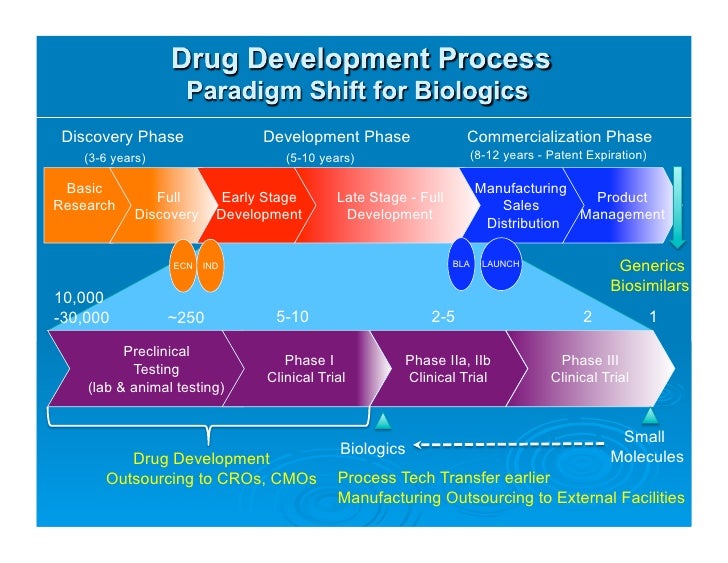 As Process Development And Manufacturing Cmc For Biologics Developm
As Process Development And Manufacturing Cmc For Biologics Developm
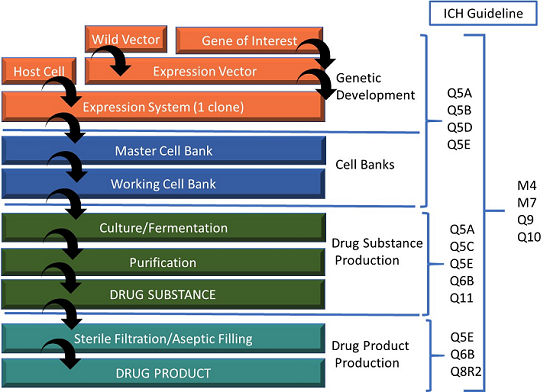 Considerations For Biologic Drug Substance And Drug Product Testing
Considerations For Biologic Drug Substance And Drug Product Testing
 Implementation Of A Platform Approach For Early Biologics Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Implementation Of A Platform Approach For Early Biologics Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Biosimilar Development Approval Of Biosimilar Medicines Through Totality Of The Evidence
Biosimilar Development Approval Of Biosimilar Medicines Through Totality Of The Evidence
 Biologics Solutions Lab Informatics Platform For Biologics
Biologics Solutions Lab Informatics Platform For Biologics
 Overcoming The Challenges In Biologics Development And Quality Control
Overcoming The Challenges In Biologics Development And Quality Control
 Phases Of Drug Development Process Drug Discovery Process Northeast Biolab
Phases Of Drug Development Process Drug Discovery Process Northeast Biolab
 Graphic Illustrating The Traditional Drug Development Process Versus A Download Scientific Diagram
Graphic Illustrating The Traditional Drug Development Process Versus A Download Scientific Diagram
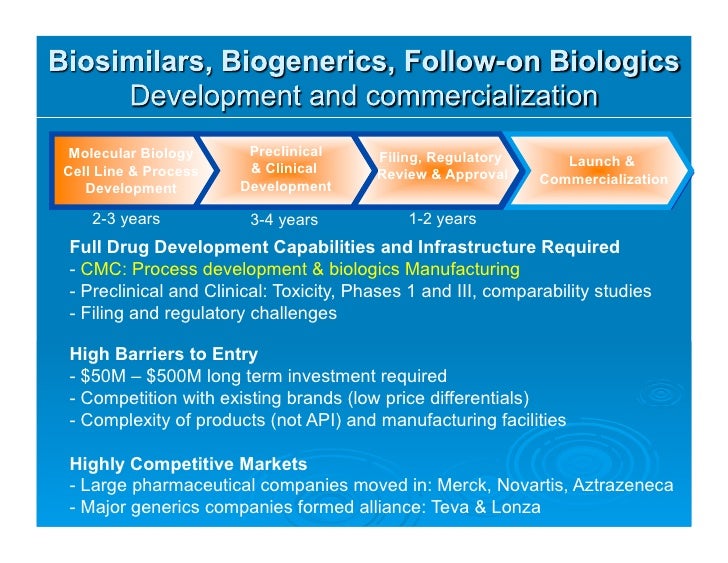 As Process Development And Manufacturing Cmc For Biologics Developm
As Process Development And Manufacturing Cmc For Biologics Developm
 Quality By Design For Monoclonal Antibodies Part 1 Establishing The Foundations For Process Development Bioprocess Internationalbioprocess International
Quality By Design For Monoclonal Antibodies Part 1 Establishing The Foundations For Process Development Bioprocess Internationalbioprocess International
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 9780470571224 Pse500
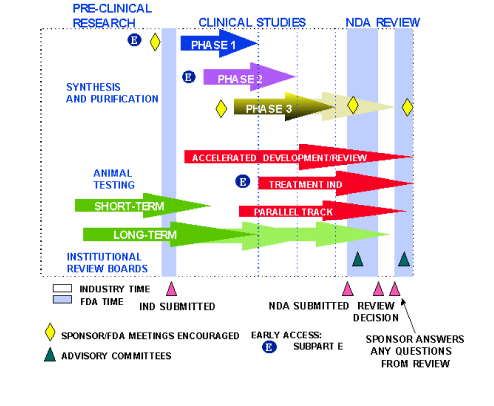
 Learning About Biosimilars Sagent Biosimilars
Learning About Biosimilars Sagent Biosimilars
 The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
The Biologics Manufacturing Process And The Manufacturing Steps That Download Scientific Diagram
 Bio Pharma Product Testing Services Eurofins Scientific
Bio Pharma Product Testing Services Eurofins Scientific
 Phases Of Drug Development Process Drug Discovery Process Northeast Biolab
Phases Of Drug Development Process Drug Discovery Process Northeast Biolab
 Faster Drug Approval Process Will Boost The Biologics Market
Faster Drug Approval Process Will Boost The Biologics Market
 Biosimilar Development Biosimilar Biological Products Development Applications
Biosimilar Development Biosimilar Biological Products Development Applications
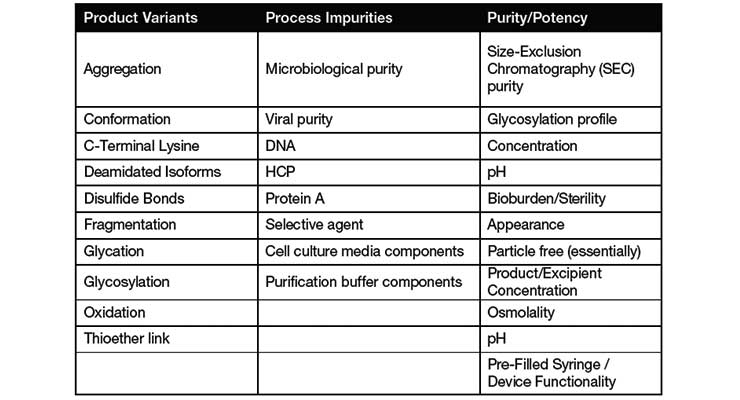 Process Validation In Biologics Development Contract Pharma
Process Validation In Biologics Development Contract Pharma
 As Process Development And Manufacturing Cmc For Biologics Developm
As Process Development And Manufacturing Cmc For Biologics Developm
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctgfskrgmf3m31n6fopwhkf Ygoggcszudo Szl4xwacjsf1v2m Usqp Cau
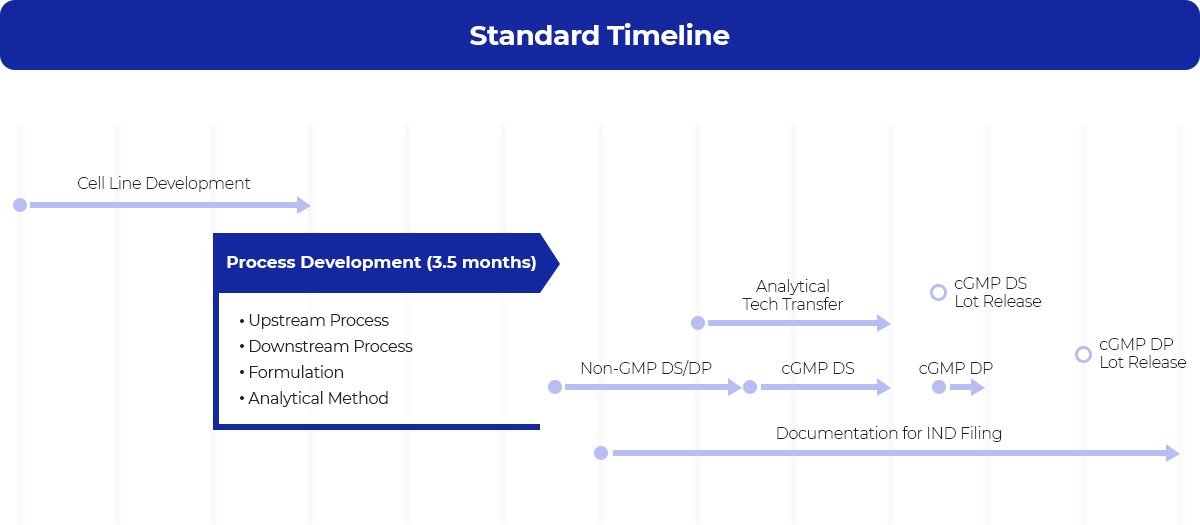 Process Development Cdo Our Services Samsung Biologics
Process Development Cdo Our Services Samsung Biologics
 Drug Discovery Development And Deployment Maps National Center For Advancing Translational Sciences
Drug Discovery Development And Deployment Maps National Center For Advancing Translational Sciences
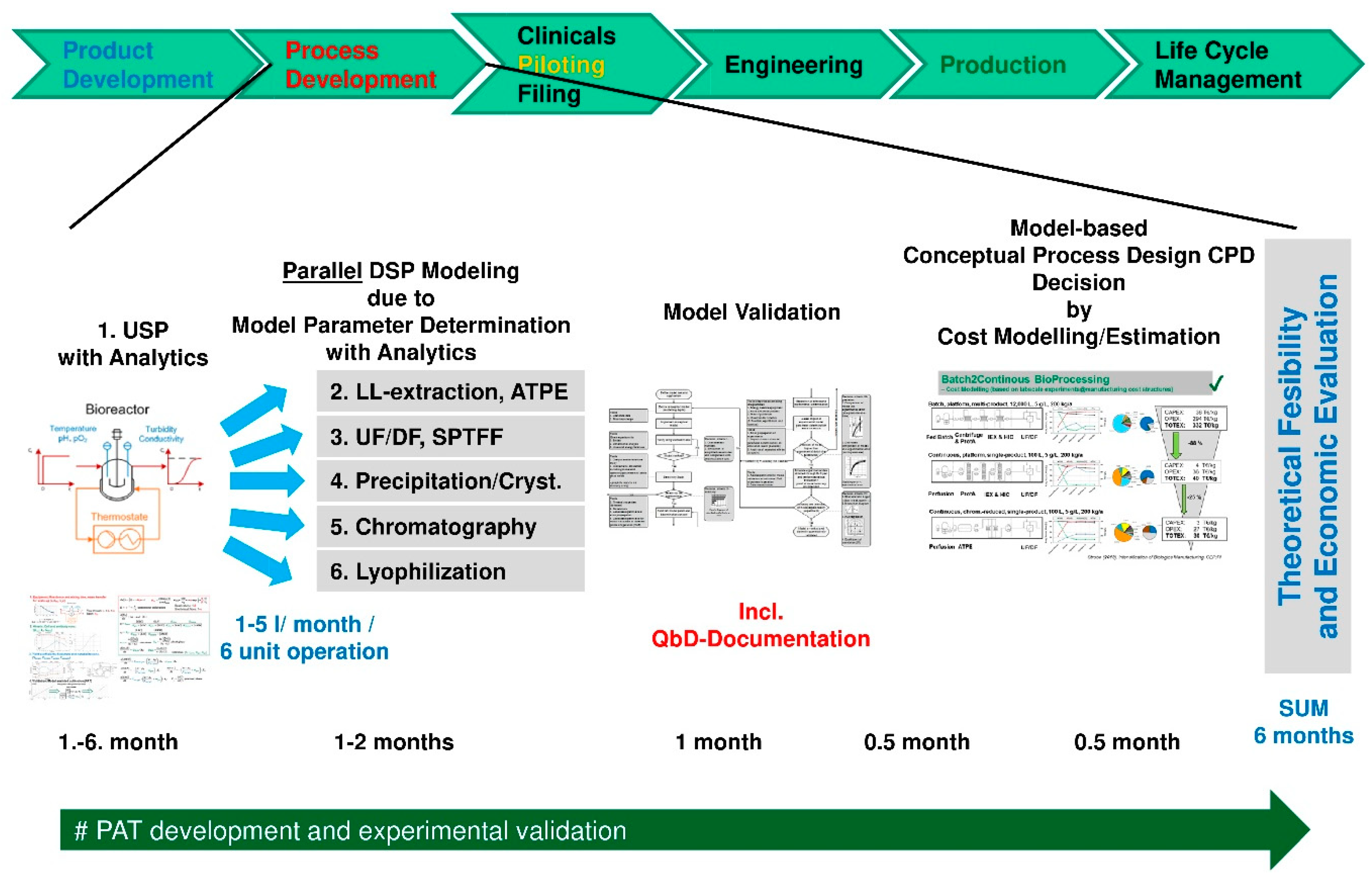 Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
Processes Free Full Text Accelerating Biologics Manufacturing By Modeling Or Is Approval Under The Qbd And Pat Approaches Demanded By Authorities Acceptable Without A Digital Twin Html
 Biosimilar Development Biosimilar Biological Products Development Applications
Biosimilar Development Biosimilar Biological Products Development Applications
Biologics Solutions Lab Informatics Platform For Biologics
 Product Stability Testing Developing Methods For New Biologics And Emerging Marketsbioprocess International
Product Stability Testing Developing Methods For New Biologics And Emerging Marketsbioprocess International
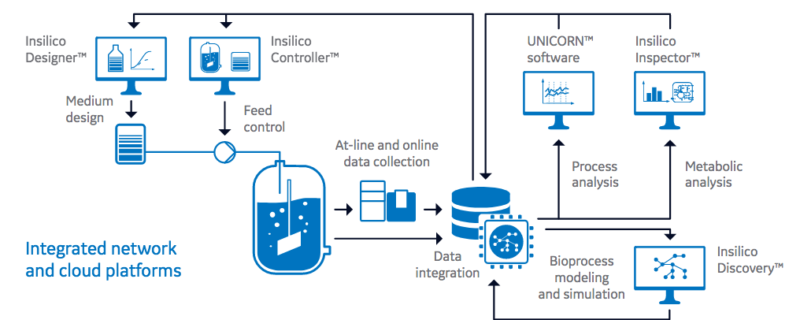 Implementing Digital Biomanufacturing In Process Development
Implementing Digital Biomanufacturing In Process Development
 Bio Pharma Product Testing Services Eurofins Scientific
Bio Pharma Product Testing Services Eurofins Scientific
 Guidelines For Biotechs Managing Risks In Biological Drug Development
Guidelines For Biotechs Managing Risks In Biological Drug Development
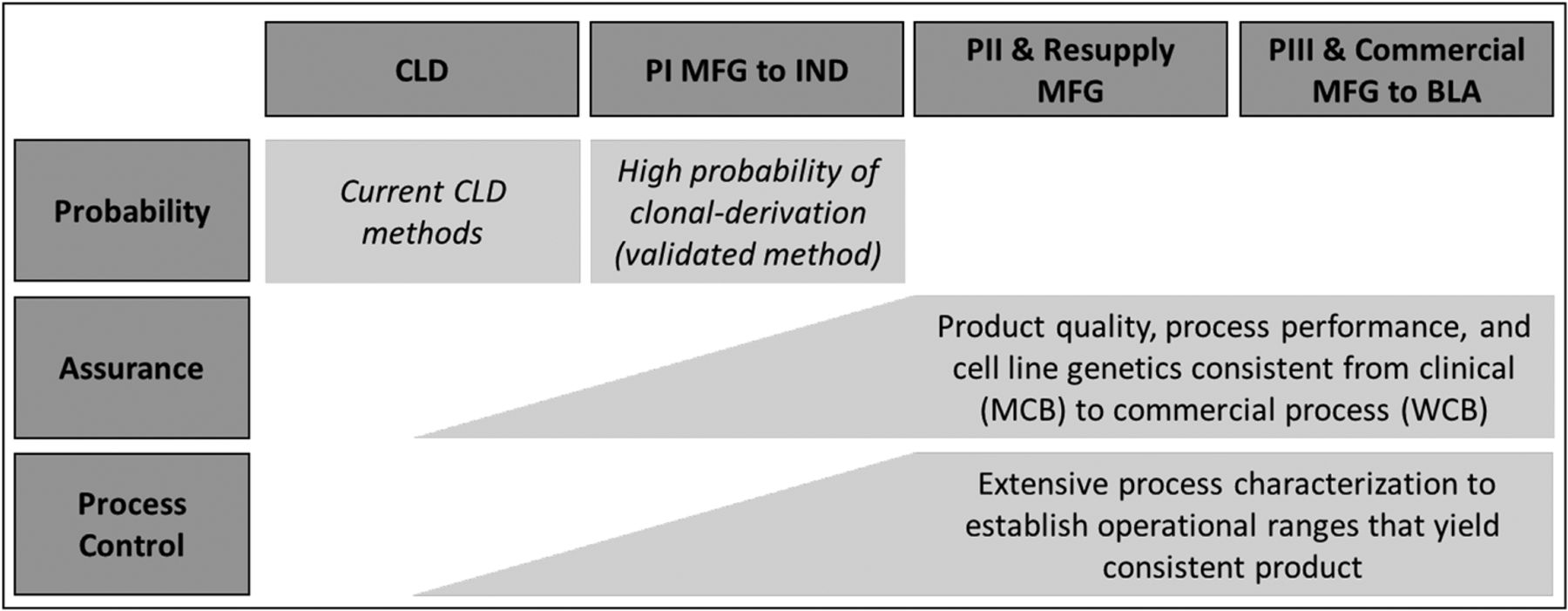 Advancing Biologics Development Programs With Legacy Cell Lines Advantages And Limitations Of Genetic Testing For Addressing Clonality Concerns Prior To Availability Of Late Stage Process And Product Consistency Data Pda Journal
Advancing Biologics Development Programs With Legacy Cell Lines Advantages And Limitations Of Genetic Testing For Addressing Clonality Concerns Prior To Availability Of Late Stage Process And Product Consistency Data Pda Journal
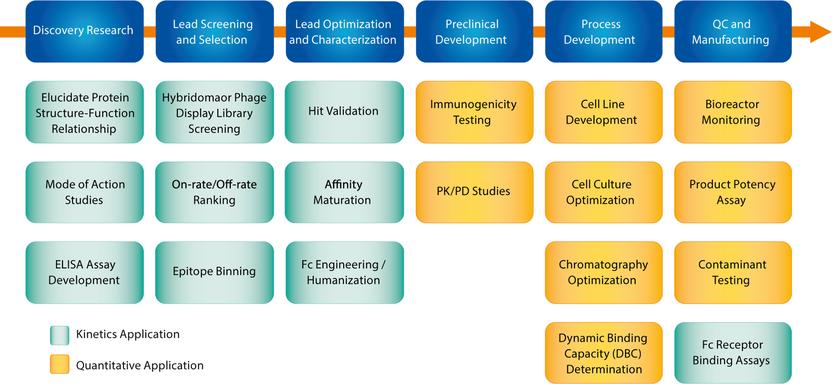 Advance Lot Release And In Process Testing Of Biologics In Qc Comprehensive Technical And Service Support For Octet Users In Gxp Environments
Advance Lot Release And In Process Testing Of Biologics In Qc Comprehensive Technical And Service Support For Octet Users In Gxp Environments
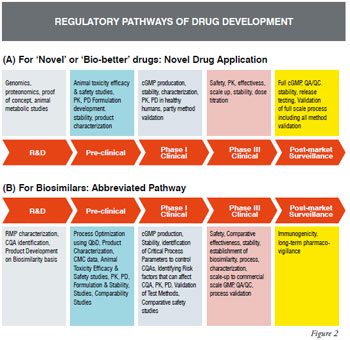 Developing Robust Approaches To The Effective Characterisation Of Biosimilars
Developing Robust Approaches To The Effective Characterisation Of Biosimilars
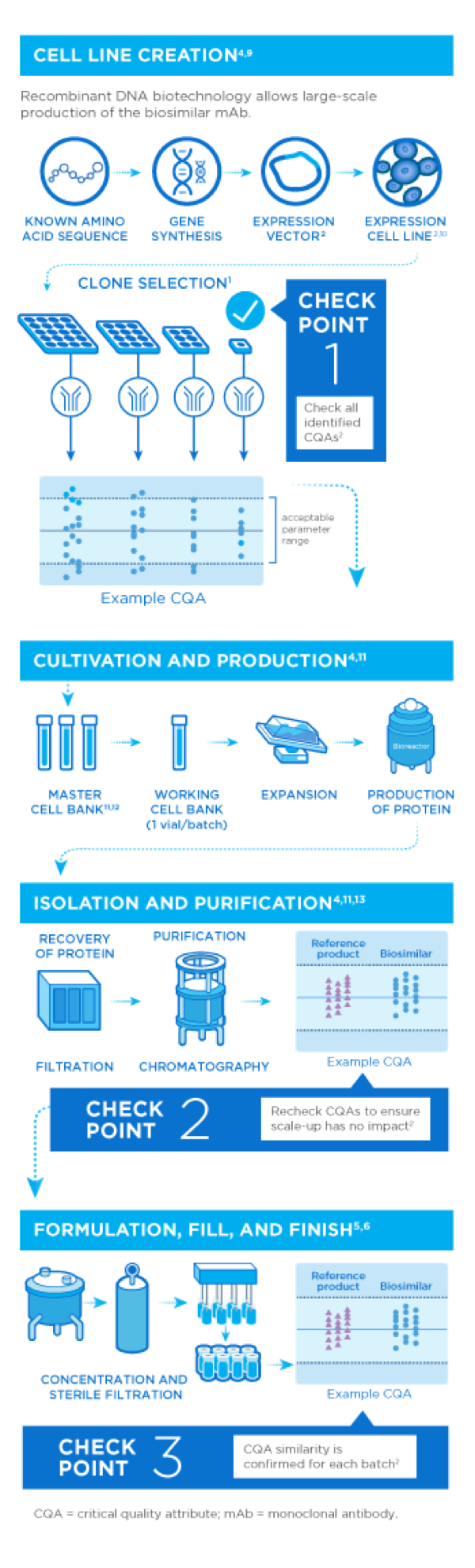 Next Generation Biologics Manufacturing Amgen Biosimilars
Next Generation Biologics Manufacturing Amgen Biosimilars
Biologics Businesses Annual Report 2018
 Pure Play Cdmo Cmab Biopharma Suzhou Inc
Pure Play Cdmo Cmab Biopharma Suzhou Inc
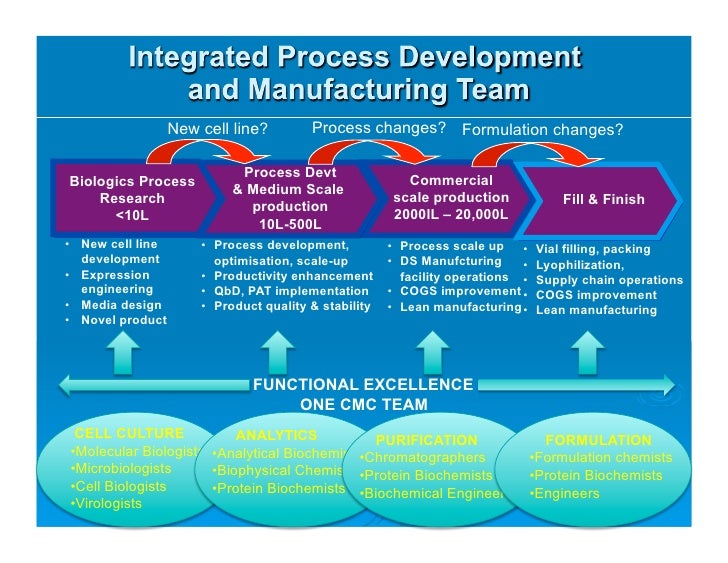 As Process Development And Manufacturing Cmc For Biologics Developm
As Process Development And Manufacturing Cmc For Biologics Developm
 Integrated Solutions For Biologics Formulation And Drug Product Development Bioprocess Internationalbioprocess International
Integrated Solutions For Biologics Formulation And Drug Product Development Bioprocess Internationalbioprocess International
An Efficient Analytical Development Strategy For Rapid Development Of Biopharmaceuticals American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Streamlining The Biologics Development Process From Transient Youtube
Streamlining The Biologics Development Process From Transient Youtube
 Drug Discovery And Development Role Of Basic Biological Research Sciencedirect
Drug Discovery And Development Role Of Basic Biological Research Sciencedirect
 Balancing A Biologics Pipeline Portfolio Drug Discovery World Ddw
Balancing A Biologics Pipeline Portfolio Drug Discovery World Ddw
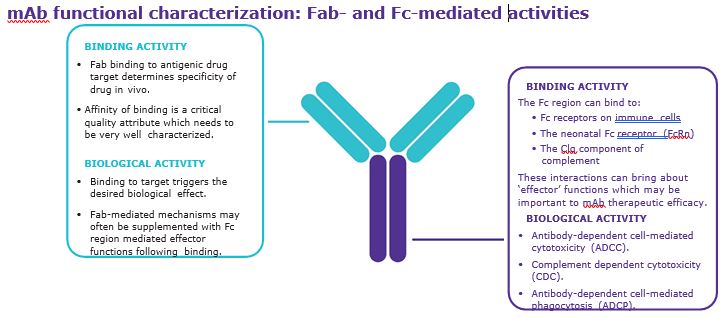 Early Product Characterization Mitigates Risks In Biologics Development
Early Product Characterization Mitigates Risks In Biologics Development
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqn Kycsg3ldxifp8qmnmk8q2o9slko9zo1slrpfiwfifxmdleb Usqp Cau
 Biologics Development How To Address Challenges In The Industry
Biologics Development How To Address Challenges In The Industry
 Biosimilar Development An Overview Trilogy Writing Consulting Gmbh
Biosimilar Development An Overview Trilogy Writing Consulting Gmbh
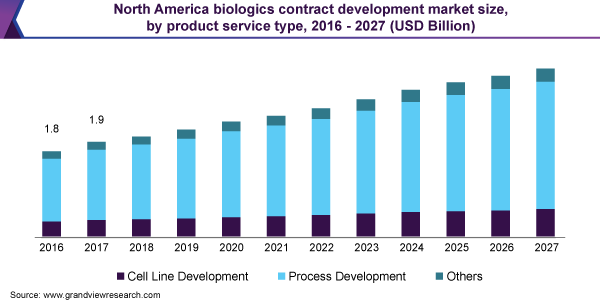 Biologics Contract Development Market Report 2020 2027
Biologics Contract Development Market Report 2020 2027
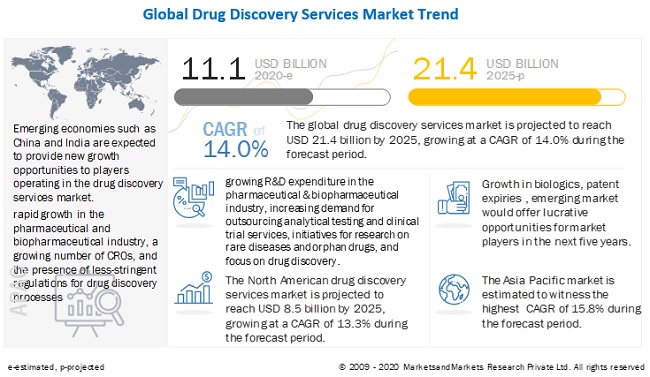 Drug Discovery Services Market Global Forecast To 2025 Marketsandmarkets
Drug Discovery Services Market Global Forecast To 2025 Marketsandmarkets
 Biopharma Services Eurofins Scientific
Biopharma Services Eurofins Scientific
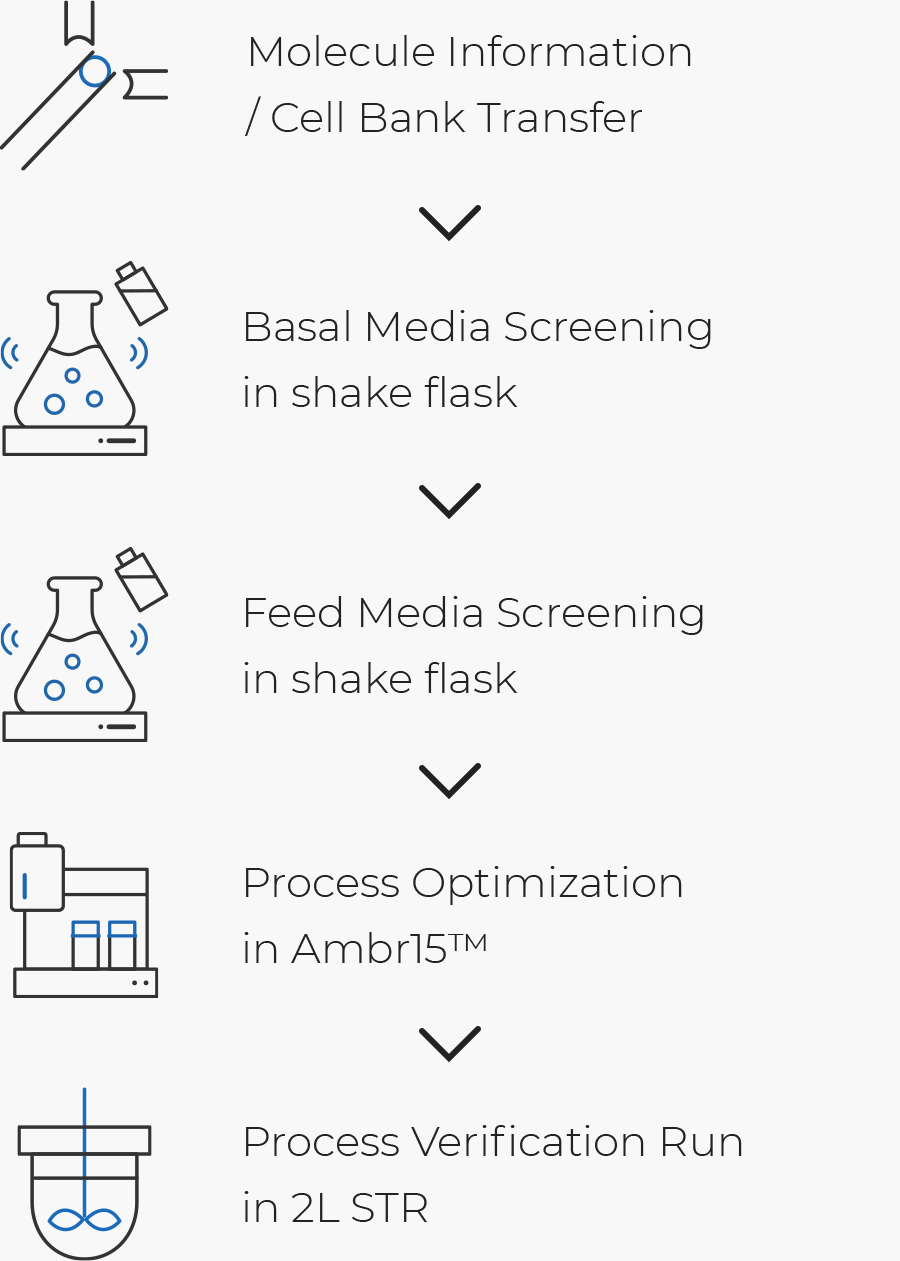 Process Development Cdo Our Services Samsung Biologics
Process Development Cdo Our Services Samsung Biologics
 The Drug Review Approval Process In Canada An Eguide Spharm Drug Regulatory Experts
The Drug Review Approval Process In Canada An Eguide Spharm Drug Regulatory Experts
Combination Products Device Development For Pharmaceutical Biologic Combination Products
Antibody Purification Process Development And Manufacturing Biopharm International
 The Process And Costs Of Drug Development Tsc
The Process And Costs Of Drug Development Tsc
 Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
 Technology Improvements To Accelerate Process Development Of Biologics American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Technology Improvements To Accelerate Process Development Of Biologics American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Drug Product Process Development Ensuring A Consistent High Quality Biologic Paperpicks Leading Content Syndication And Distribution Platform
Drug Product Process Development Ensuring A Consistent High Quality Biologic Paperpicks Leading Content Syndication And Distribution Platform
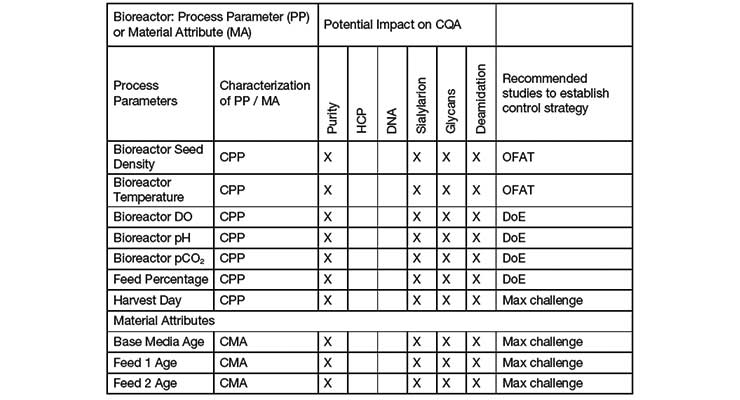 Process Validation In Biologics Development Contract Pharma
Process Validation In Biologics Development Contract Pharma
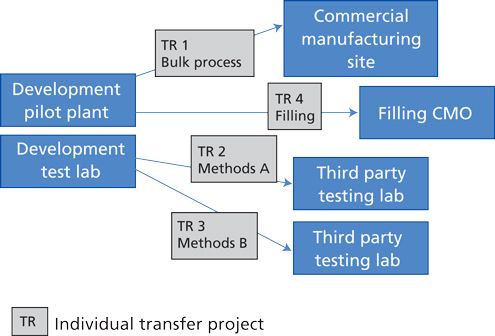 Integrating Technology Transfer And Facilities Startup For Biologics Biopharm International
Integrating Technology Transfer And Facilities Startup For Biologics Biopharm International
 As Process Development And Manufacturing Cmc For Biologics Development An Overview 26 Nov09
As Process Development And Manufacturing Cmc For Biologics Development An Overview 26 Nov09
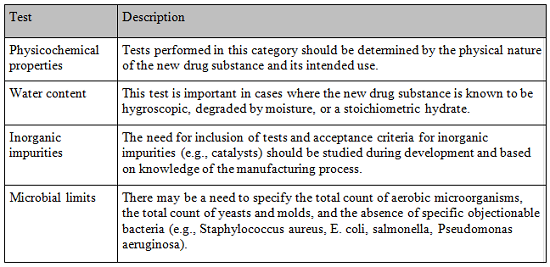 Considerations For Biologic Drug Substance And Drug Product Testing
Considerations For Biologic Drug Substance And Drug Product Testing
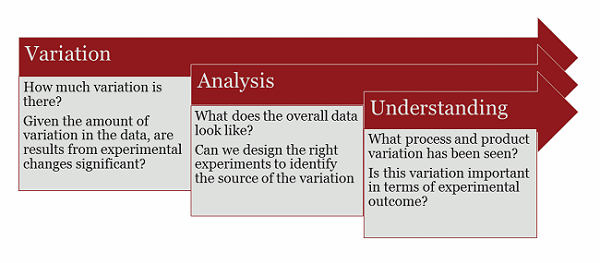 Early Implementation Of Quality By Design For Biological Product Development
Early Implementation Of Quality By Design For Biological Product Development
 Biopharmaceutical Process Development Trends Challenges Opportunities
Biopharmaceutical Process Development Trends Challenges Opportunities
 Drug Discovery And Development Role Of Basic Biological Research Sciencedirect
Drug Discovery And Development Role Of Basic Biological Research Sciencedirect
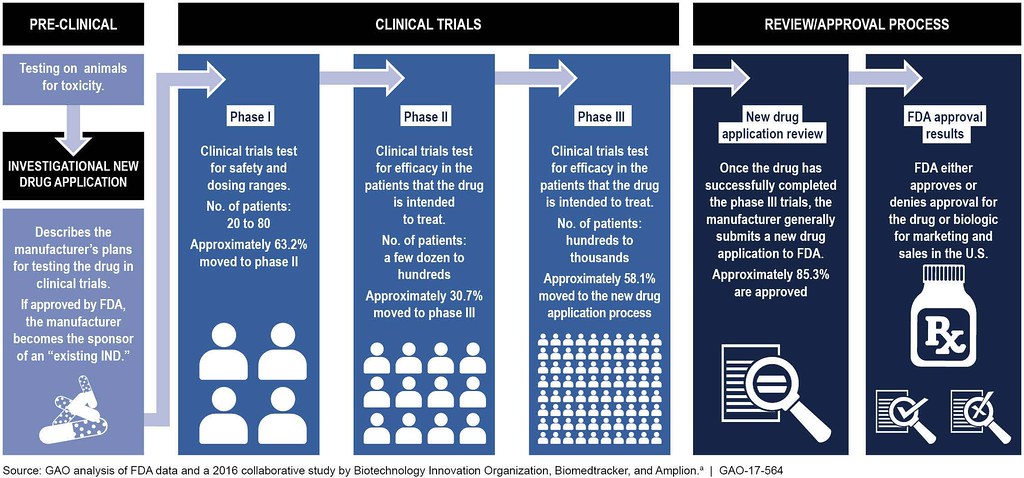 6 2 The Drug Development Process Biology Libretexts
6 2 The Drug Development Process Biology Libretexts
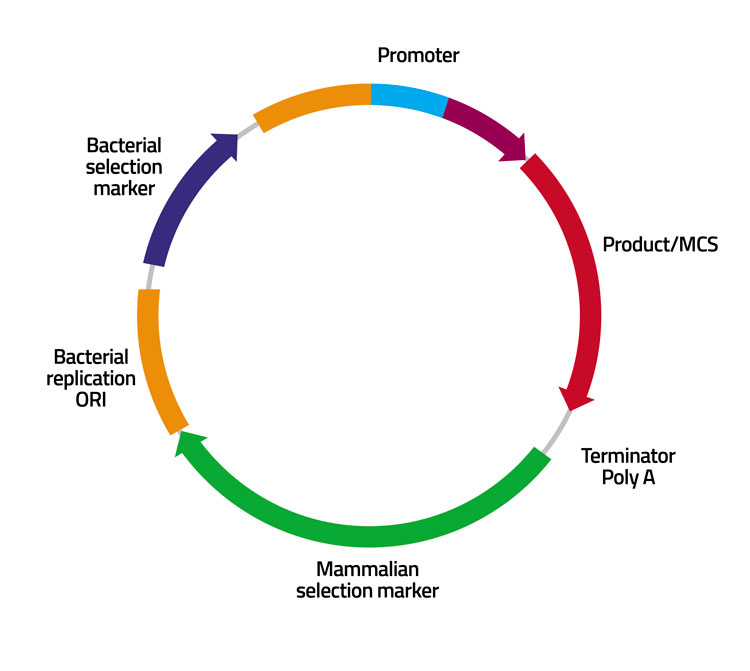 Cell Line Development Biologics Development From Early Phase To Ind
Cell Line Development Biologics Development From Early Phase To Ind
 Developing Analytical Instrumentation For The Biopharmaceutical Industry
Developing Analytical Instrumentation For The Biopharmaceutical Industry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcruuuregu89o0q197rip Pi4cpb0jvjpiklrkycsmy 7skmzkz Usqp Cau
 Early Product Characterization Mitigates Risks In Biologics Development
Early Product Characterization Mitigates Risks In Biologics Development
 The Process And Costs Of Drug Development Tsc
The Process And Costs Of Drug Development Tsc
 Biosimilars And Drug Development In Allergic And Immunologic Diseases Journal Of Allergy And Clinical Immunology
Biosimilars And Drug Development In Allergic And Immunologic Diseases Journal Of Allergy And Clinical Immunology
 Exploring The Drug Development Process Technology Networks
Exploring The Drug Development Process Technology Networks
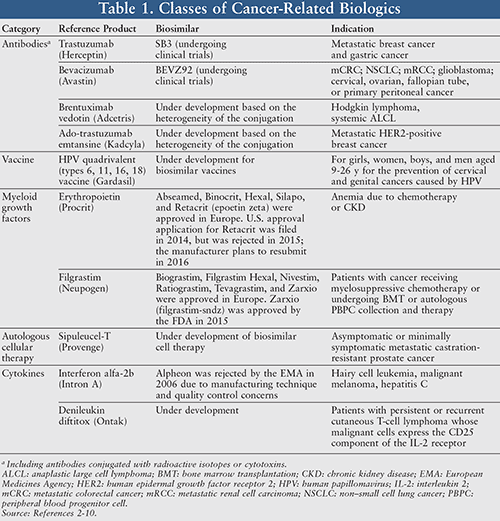 Cancer Biosimilars Regulation Challenges And Clinical Impact
Cancer Biosimilars Regulation Challenges And Clinical Impact
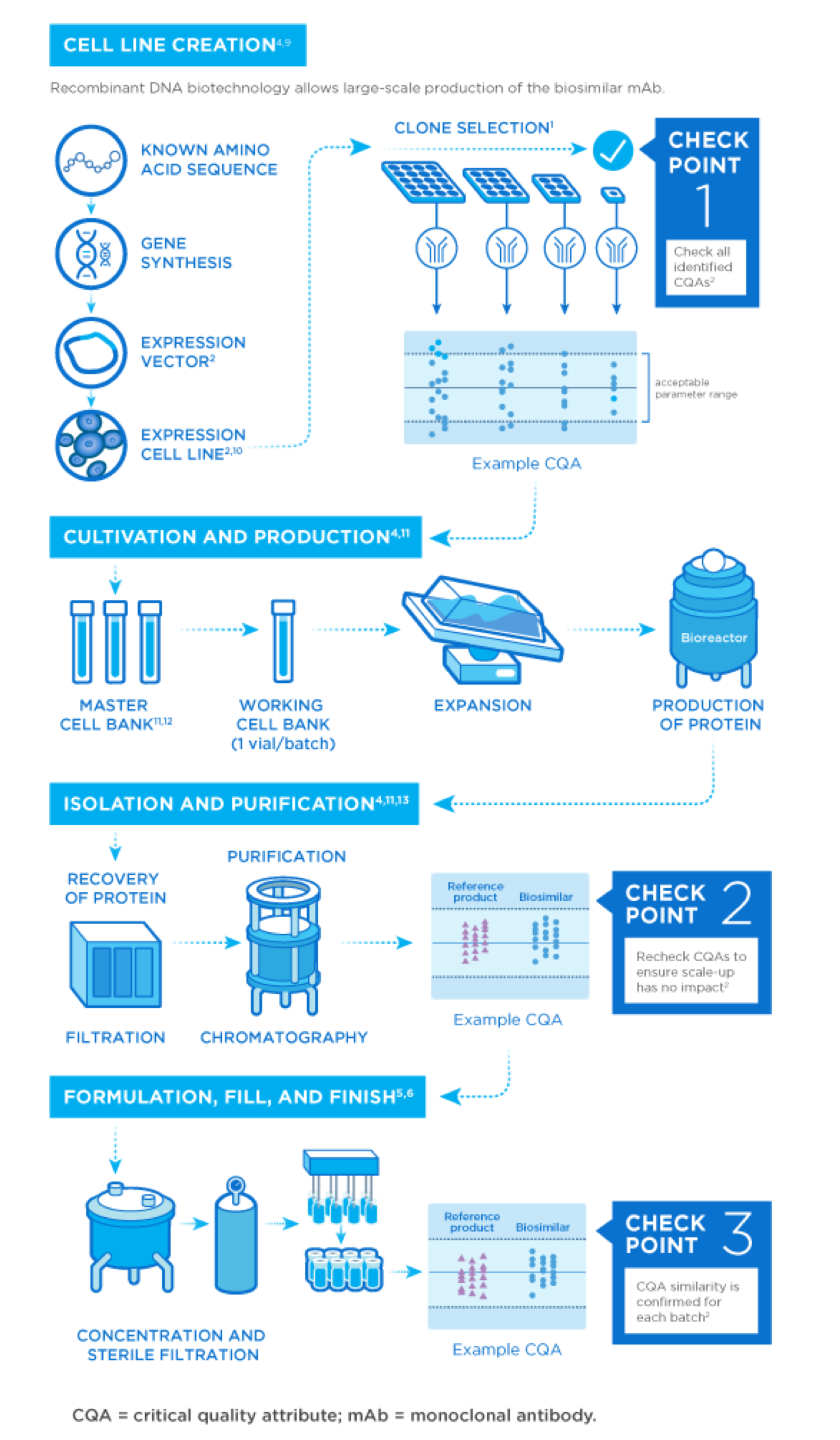 Next Generation Biologics Manufacturing Amgen Biosimilars
Next Generation Biologics Manufacturing Amgen Biosimilars
 Fully Supporting Customer Needs For Biologics Development And Manufacturing
Fully Supporting Customer Needs For Biologics Development And Manufacturing
 Overcoming The Challenges In Biologics Development And Quality Control
Overcoming The Challenges In Biologics Development And Quality Control
 The Drug Development And Approval Process Is About Much More Than The Final Okay
The Drug Development And Approval Process Is About Much More Than The Final Okay
 Process Validation For Drugs And Biologics By Grc Training Solutions Medium
Process Validation For Drugs And Biologics By Grc Training Solutions Medium
Best In Class Cell Line Development And Biologic Manufacturing Coming Together Selexis Sa
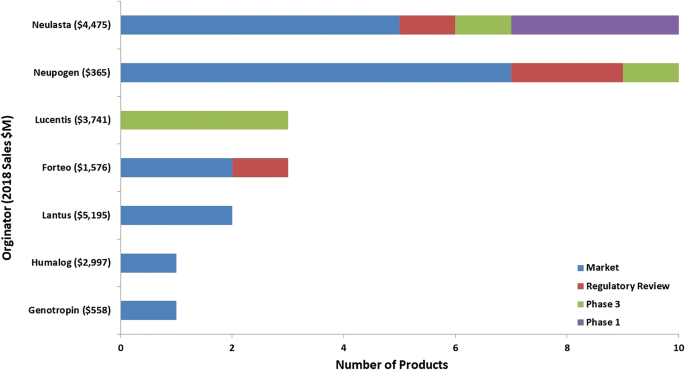 Current Perspectives On Biosimilars Springerlink
Current Perspectives On Biosimilars Springerlink
 2 Drug Development Challenges Improving And Accelerating Therapeutic Development For Nervous System Disorders Workshop Summary The National Academies Press
2 Drug Development Challenges Improving And Accelerating Therapeutic Development For Nervous System Disorders Workshop Summary The National Academies Press
 Analytical Testing Development Qualification Validation
Analytical Testing Development Qualification Validation
 An Overview Of Biologics Manufacturing Processes And Things To Consid
An Overview Of Biologics Manufacturing Processes And Things To Consid
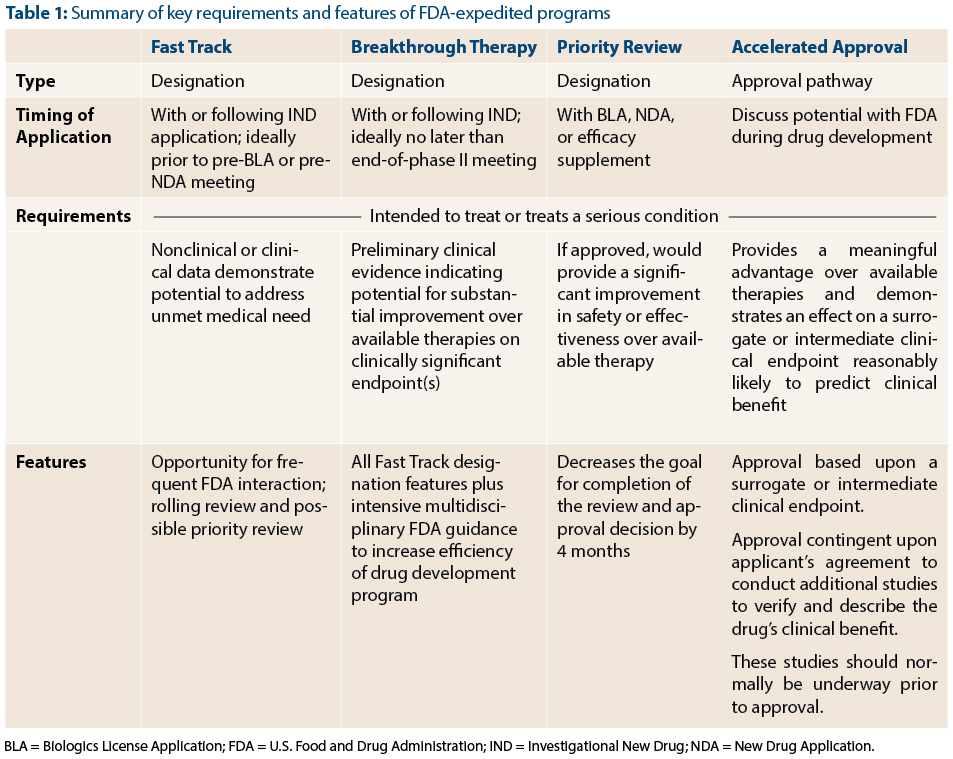 Fda Programs To Expedite Drug And Biologic Product Development The Asco Post
Fda Programs To Expedite Drug And Biologic Product Development The Asco Post
 Drug Development Process And Novel Drugs Approved By Fda For 2017 18 Bentham Science
Drug Development Process And Novel Drugs Approved By Fda For 2017 18 Bentham Science
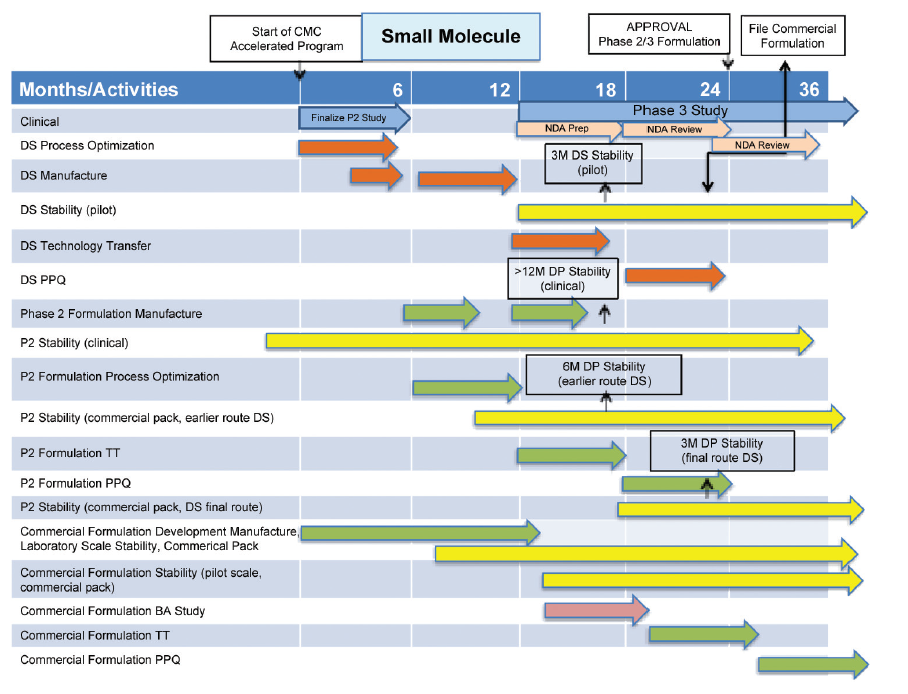 Cmc Considerations When A Drug Development Project Is Assigned Breakthrough Therapy Status Pharmaceutical Engineering
Cmc Considerations When A Drug Development Project Is Assigned Breakthrough Therapy Status Pharmaceutical Engineering
Fda S Drug Review Process All About Drugs
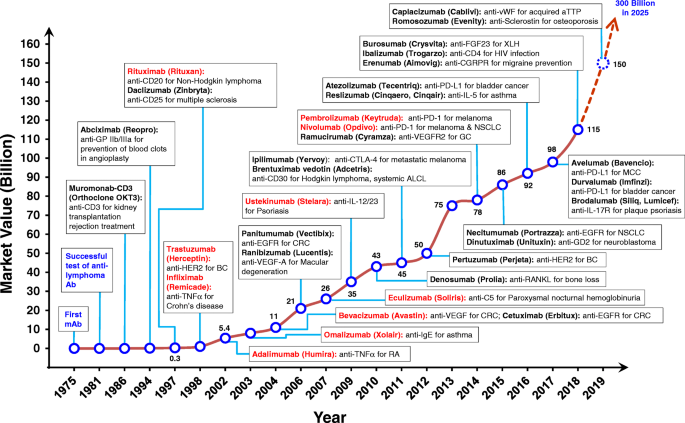 Development Of Therapeutic Antibodies For The Treatment Of Diseases Journal Of Biomedical Science Full Text
Development Of Therapeutic Antibodies For The Treatment Of Diseases Journal Of Biomedical Science Full Text
High Throughput Screening In Biologics Process Development With A Minimum Of Material
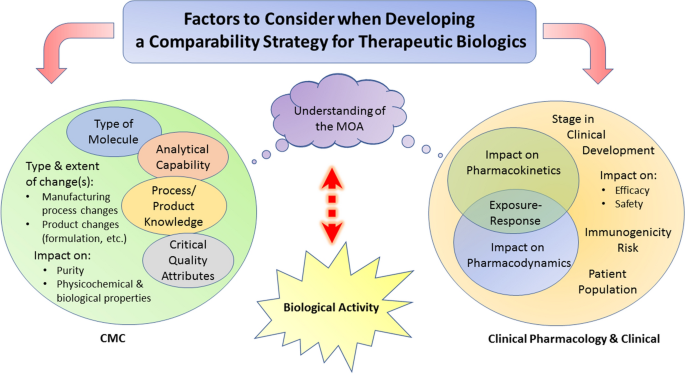 Comparability Considerations And Challenges For Expedited Development Programs For Biological Products Springerlink
Comparability Considerations And Challenges For Expedited Development Programs For Biological Products Springerlink
 Advanced Process Development Using Automated Micro Bioreactors Shortens Timeline
Advanced Process Development Using Automated Micro Bioreactors Shortens Timeline
 Department Of Health What Are Biosimilar Medicines
Department Of Health What Are Biosimilar Medicines
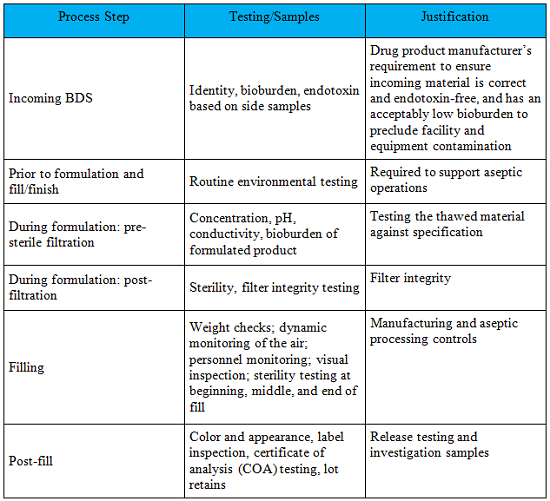 Considerations For Biologic Drug Substance And Drug Product Testing
Considerations For Biologic Drug Substance And Drug Product Testing
 Reviewed by guruku
on
February 17, 2021
Rating:
Reviewed by guruku
on
February 17, 2021
Rating:






No comments: